Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
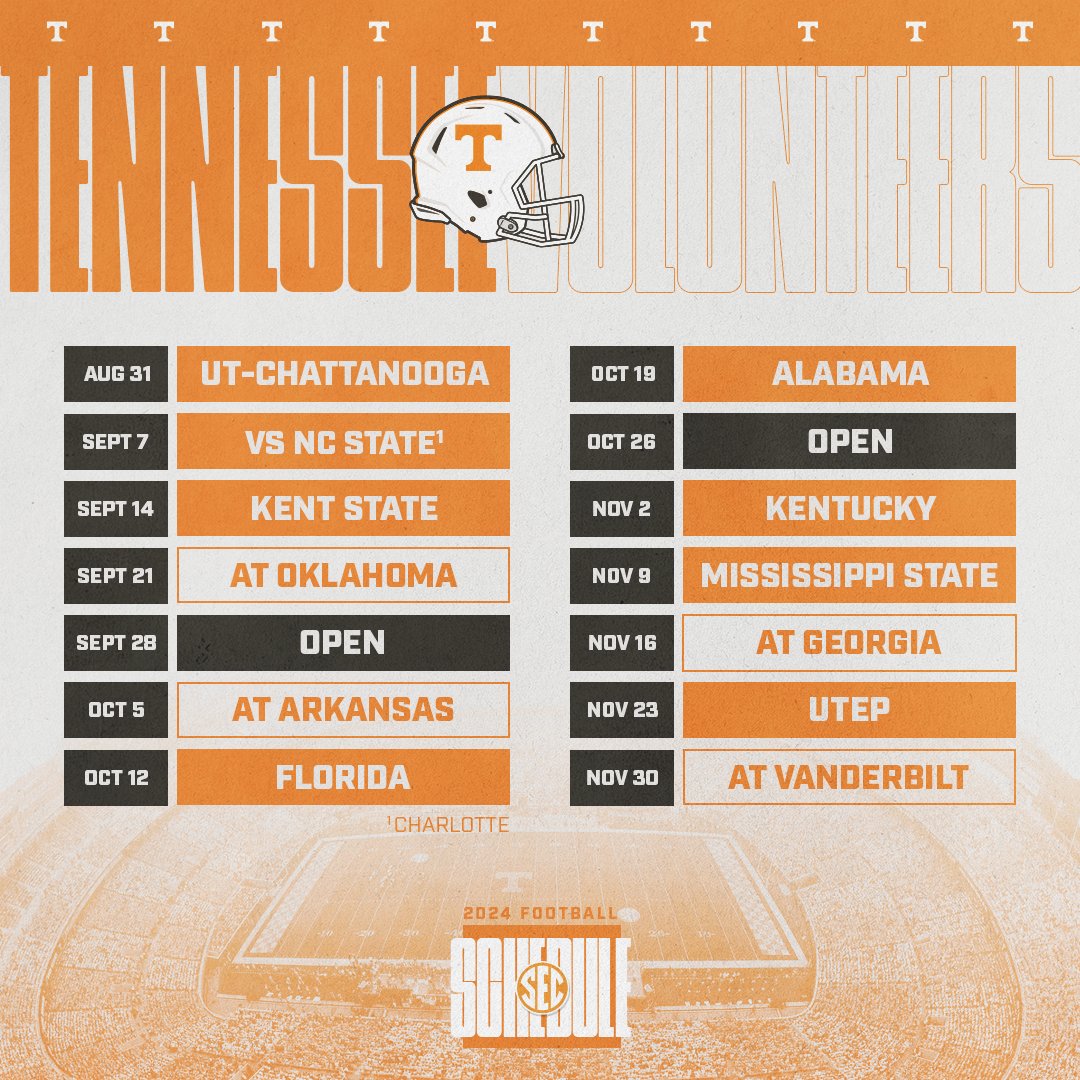 | 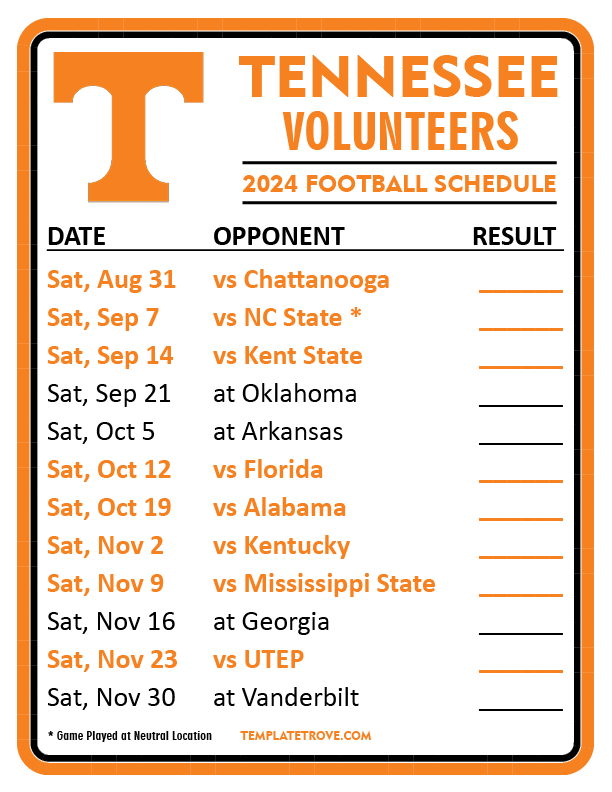 |
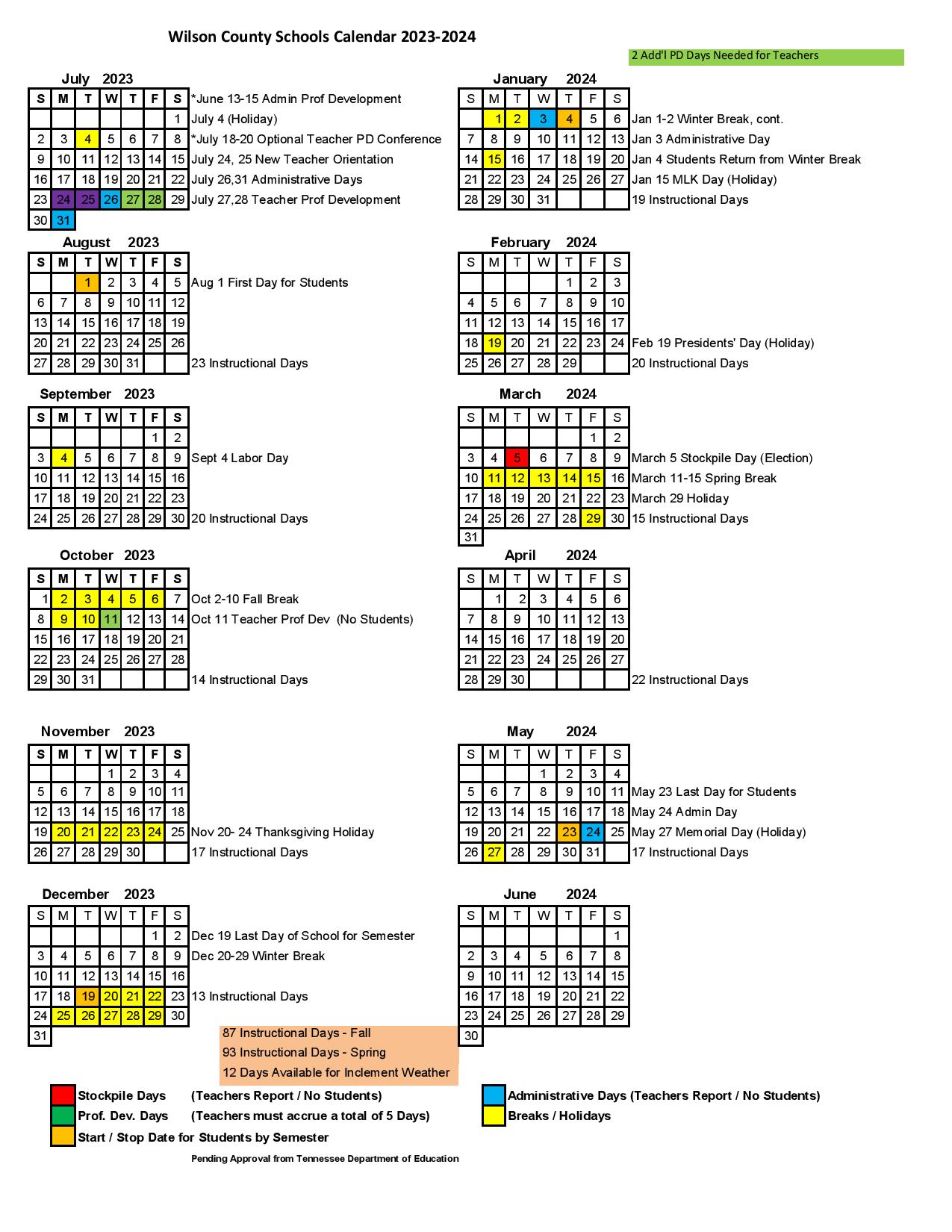
In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). The Tennessee (TN) Controlled Substance Monitoring Database (CSMD) is a Prescription Drug Monitoring Program (PDMP) designed to provide healthcare practitioners with a comprehensive view of a patient’s history of controlled substance prescriptions. The CSMD collects prescriptions from pharmacies licensed by the Tennessee Board of Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. (a) Schedule V consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (b) Narcotic drugs containing non-narcotic active medicinal ingredients. Tennessee Departmen t of Health Key Points Related t o the Drug Epidemic The Tennessee (TN) Controlled Substance Monitoring Database (CSMD) is a Prescription Drug Monitoring Program (PDMP) designed to provide healthcare practitioners with a comprehensive view of a patient’s history of controlled substance prescriptions. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk of overdose 2010 Tennessee Code Title 39 - Criminal Offenses Chapter 17 - Offenses Against Public Health, Safety and Welfare Part 4 - Drugs 39-17-410 - Controlled substances in Schedule III. 39-17-410. Controlled substances in Schedule III. 2010 Tennessee Code Title 39 - Criminal Offenses Chapter 17 - Offenses Against Public Health, Safety and Welfare Part 4 - Drugs 39-17-414 - Controlled substances in Schedule V. 39-17-414. Controlled substances in Schedule V. Effective July 1, 2018, all gabapentin products became Schedule V controlled substances in the state of Tennessee. Gabapentin is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin is often used to potentiate the effects of opioids and potentially increases the risk (1) Schedule I consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this rule. Each drug or > gabapentin_schedule_v175X100 June 21, 2018 The Tennessee Pharmacists Association (TPA) is the only membership organization serving all pharmacy professionals, student pharmacists, and pharmacy technicians in Tennessee, in all practice categories and across the state Justia › U.S. Law › U.S. Codes and Statutes › Tennessee Code › 2021 Tennessee Code › Title 39 - Criminal Offenses › Chapter 17 - Offenses Against Public Health, Safety and Welfare › Part 4 - Drugs › § 39-17-414. Controlled Substances in Schedule v In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in Tennessee classifies gabapentin as a Schedule V controlled substance under state law, a designation enacted in 2018 through an amendment to the Tennessee Controlled Substances Act. This made Tennessee one of the first states to impose such restrictions, driven by concerns about gabapentin’s role in opioid abuse. Justia Free Databases of U.S. Laws, Codes & Statutes. 2024 Tennessee Code Title 39 - CRIMINAL OFFENSES (§§ 39-1-101 — 39-17-1812) Chapter 17 - OFFENSES AGAINST PUBLIC HEALTH, SAFETY AND WELFARE (§§ 39-17-101 — 39-17-1812) Part 4 - DRUGS (§§ 39-17-401 — 39-17-456) Section 39-17-410 - Controlled substances in Schedule III Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. In general, medications classified as Schedule V (Schedule 5) controlled substances are considered to have the lowest potential for abuse compared to other On June 21, TPA released a member resource for pharmacists and pharmacy professionals, to assist practices regarding the scheduling of gabapentin as it becomes a schedule V controlled substance. Any pharmacy, or licensed healthcare practitioner, who has a DEA number and dispenses gabapentin in (or into) Tennessee must report to the database daily (but no later than the close of business on the following business day) each controlled substance they have dispensed over the last 24 hours. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |