Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
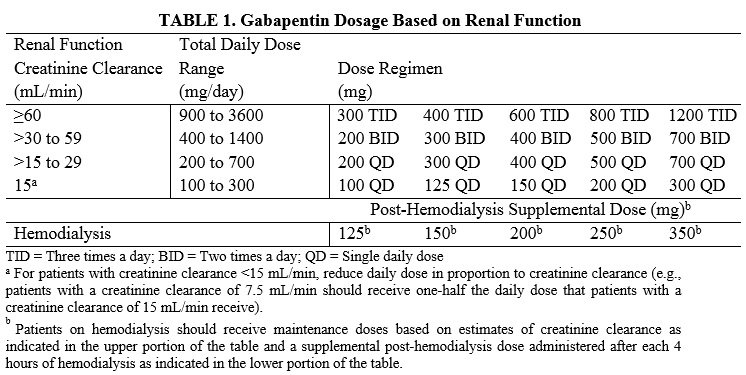
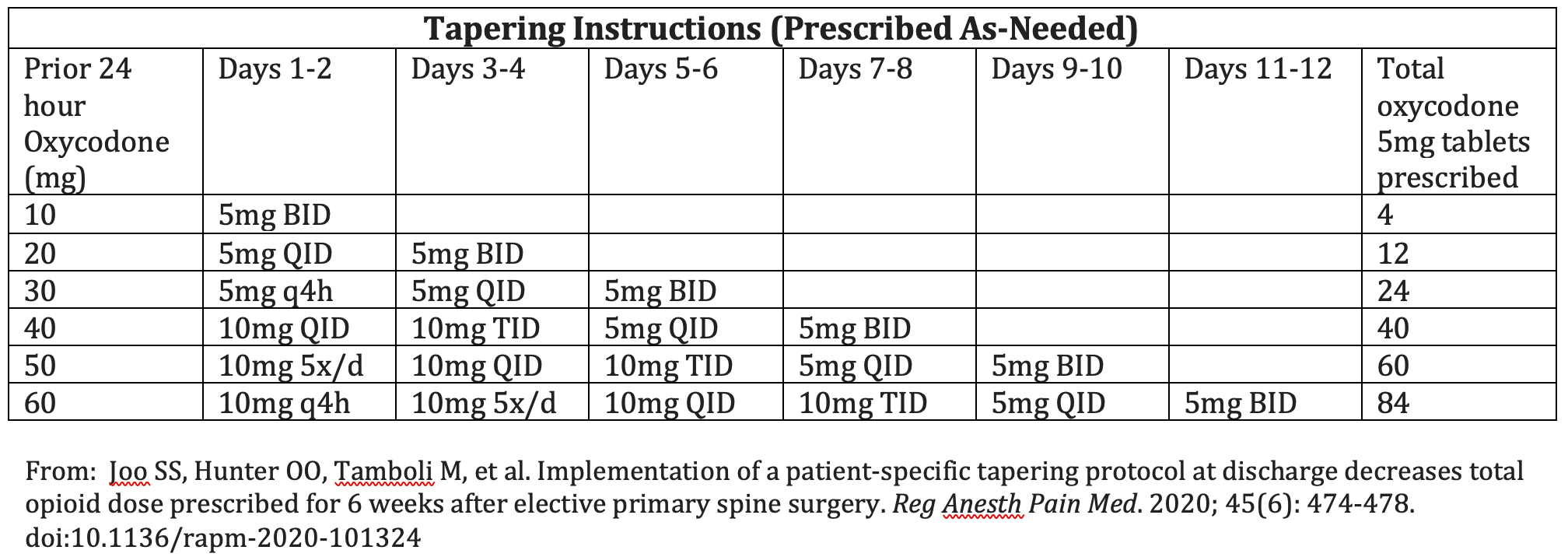
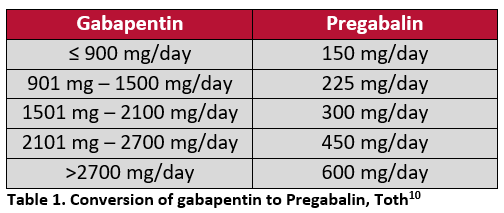
The reclassification of pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019 will affect vets. These schedule 3 drugs: will be exempt from safe Gabapentin is licensed for the treatment of peripheral neuropathic pain such as painful diabetic neuropathy and postherpetic neuralgia in adults [ABPI, 2020a].However, the National Institute for Health and Care Excellence (NICE) recommends gabapentin as a first-line treatment option for adults with all neuropathic pain (except trigeminal neuralgia) [NICE, 2019a]. Gabapentin and pregabalin are in schedule 3, but not in the “must be kept locked in a CD cabinet” schedule 3 list. Hence, they do not need to be kept in the CD cabinet, recorded in the CD register or given with a witness. Gabapentinoids have been reclassified as Schedule 3 CDs under the Misuse of Drugs Regulations 2001 and Class C controlled substances under the Misuse of Drugs Act 1971 from st1 April 2019. Following concerns about abuse, both gabapentinoids were reclassified as Schedule 3 Controlled Drugs in April 2019. Key Messages. Discuss realistic expectations of therapy with patients who experience chronic pain – a realistic goal of treatment would be a 30% improvement in pain and/or a significant improvement in functional ability MHRA/CHM advice: Gabapentin (Neurontin®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. From 1st April 2019, amendments to the Misuse of Drugs Regulations 2001 and the Safe Custody Regulations 1973 come into force which mean that pregabalin and gabapentin will be reclassified as Schedule 3 Controlled Drugs (CDs). Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. Summary Requirements for gabapentin and pregabalin from 1st April 2019 are as follows: Controlled Drug Prescription requirements Prescription validity Gabapentin: reduce the daily dose at a maximum rate of 300mg every four days. Examples of withdrawal schedules Example withdrawal schedule for a dose of Pregabalin 150mg bd The ACMD recommended that gabapentin and pregabalin be controlled as Class C drugs under the 1971 Act, and placed in Schedule 3 to the 2001 Regulations. From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. People reducing their opioid or gabapentinoid dose should be reviewed at least every two weeks, with assessment of pain, level of function, and signs of withdrawal. The initial consultation should be face-to-face to explain why the opioid is being reduced, but it may be possible for subsequent consultations to be conductive via telephone. Analgesic Tapering Guidelines for adult patients with persistent pain patients taking strong opioids and/or gabapentinoids. Prescribing of gabapentinoids for neuropathic pain should be reviewed in line with the criteria set out in NICE4 and should be gradually discontinued if ineffective. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. Veterinary surgeons must store CDs securely and appropriately in a suitable cabinet to prevent unauthorised access. All Schedule 2 CDs, with the exception of quinalbarbitone, as well as Schedule 3 CDs containing buprenorphine, diethylpropion, flunitrazepam, and temazepam, are legally required to be stored in a locked cabinet which is compliant with the Safe Custody Regulations. services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Background In January 2016, the Advisory Committee for the Misuse of Drugs recommended that pregabalin and gabapentin are scheduled as Sch 3 CDs within the Misuse of Drugs gabapentin and pregabalin This leaflet applies to gabapentinoid use in chronic pain only What are gabapentinoids? Gabapentinoids are medicines which can help to manage neuropathic (nerve) pain, caused by sensitive, damaged or malfunctioning nerves. They are only recommended for neuropathic type pain. Medication alone will not cure the pain. CD3 (Schedule 3 (CD No Register Exempt Safe Custody)) Gabapentin 600mg tablets Accord-UK Ltd Show Cautionary and advisory labels. Label . 3 Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Rhybudd: Gall y feddyginiaeth hon eich gwneud yn gysglyd. £543,385 was spent on pregabalin and gabapentin.[epact2 2021] There is published evidence that both gabapentin and pregabalin are subject to abuse and misuse. Both medicines have known psychiatric side effects including euphoria. Individuals misusing gabapentin and As of 1 April 2019, pregabalin and gabapentin are classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 (as
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |