Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
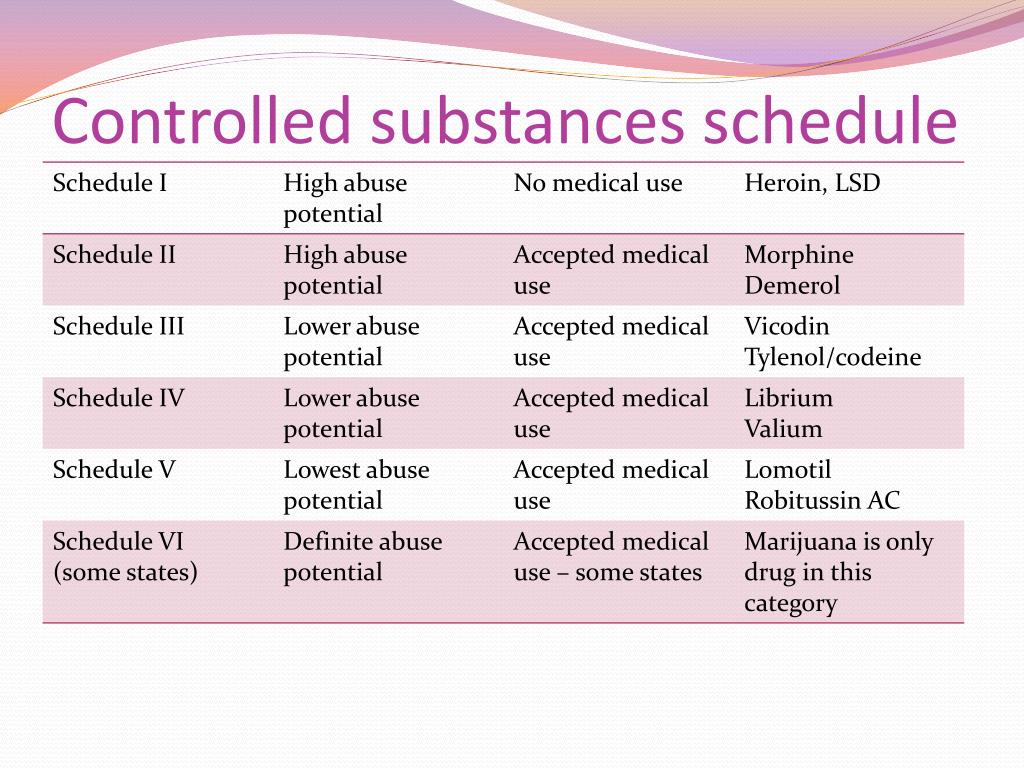
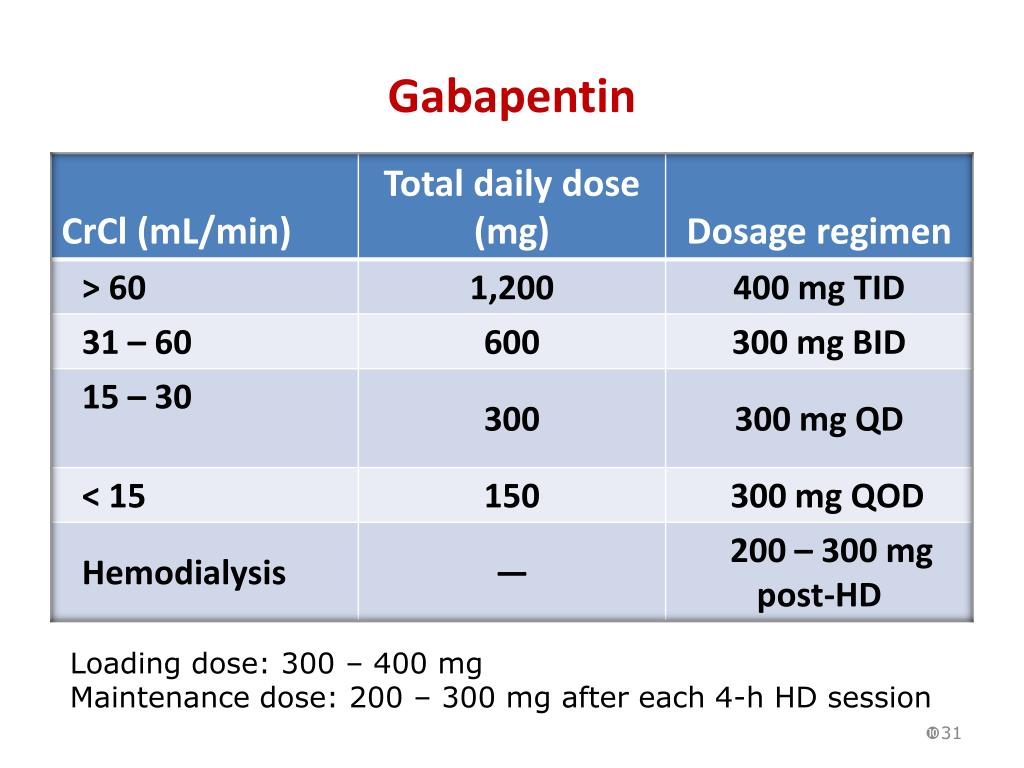
Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug nalbuphine (Schedule IV in KY, not scheduled federally) gabapentin (Scheduled V in KY, not scheduled federally) barbital, methylphenobarbital and phenobarbital (Schedule III in KY, Schedule IV federally)--Be advised--KY does not exempt ANY butalbital product from the Control Substance Act (902 KAR 55:045) Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote. For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration on the United Kingdom recently reclassifying gabapentin as a Schedule III controlled drug in April 2019.27 The U.K. classifi-cation system is similar to the U.S. system in that there are 5 schedules, but it is slightly different in that the scheduling indicates the level of control compared to potential for abuse with the U.S. system. All applicable provisions of recently passed Utah House Bill 260 C ONTROLLED SUBSTANCES. AMENDMENT S that aected UCA 58-37-4 Schedules of con trolled substances and other . licensure board regulations will apply t o Gabapentin. Please review all con trolled substance security, storage, record keeping, inventor y, Annual Review Completed for all Drug Entries on 9-15-2022 . Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. Abbreviations Used in Reference Table C.S.A Schedules I Schedule I II Schedule II III Schedule III While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Pregabalin was likely scheduled as a controlled substance owing to concerns for abuse potential, which may be attributed to higher binding affinity for voltage-activated calcium channels, greater than 90% bioavailability, regardless of dose, and more rapid absorption after administration. 12, 14 Unlike pregabalin, gabapentin bioavailability Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |