Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
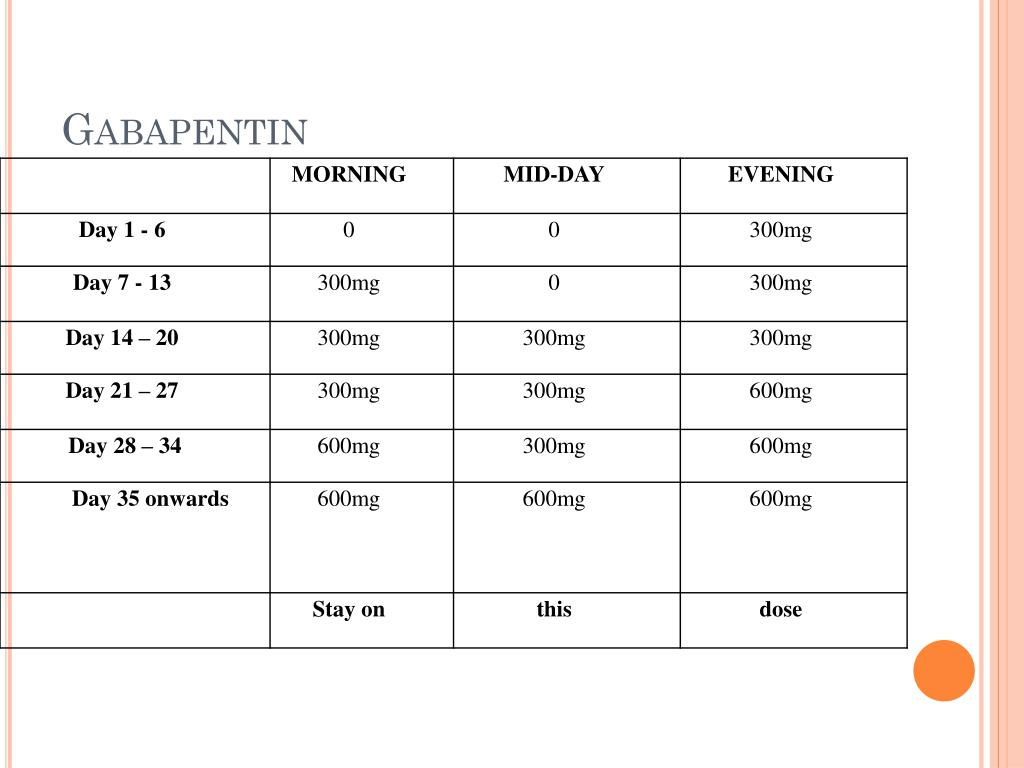
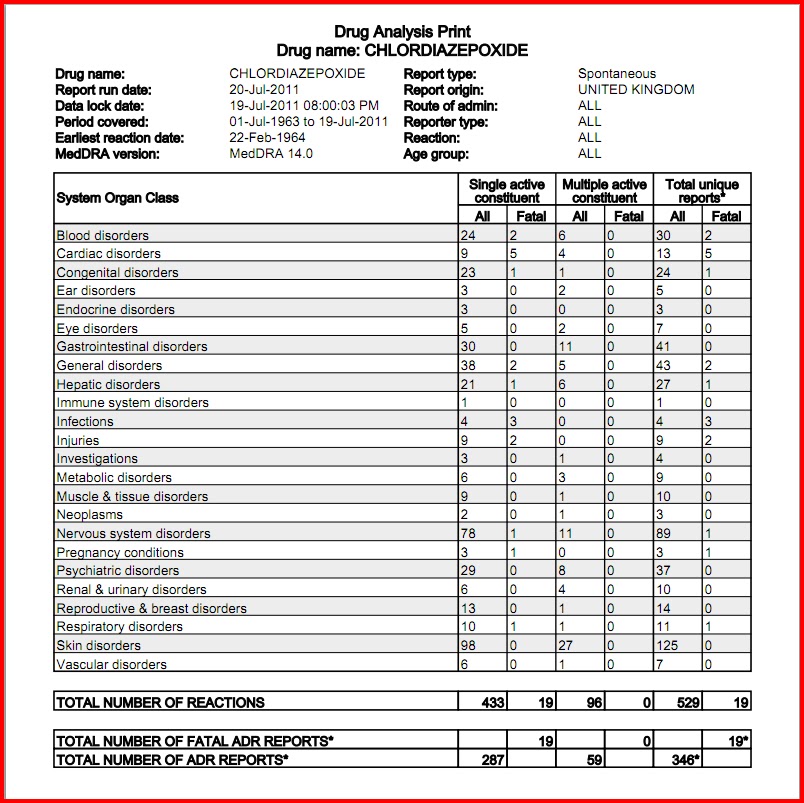
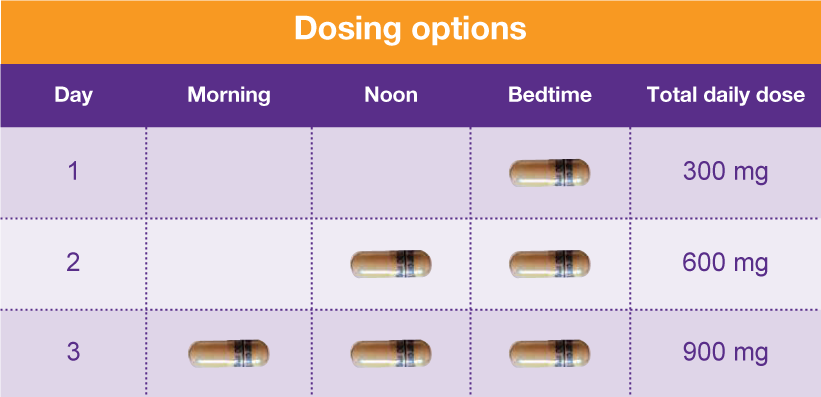
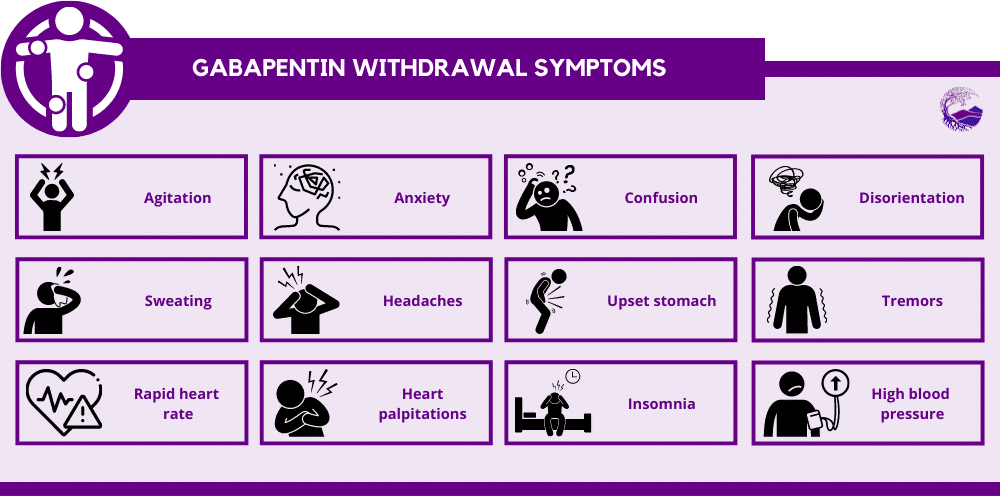
According to American Addiction Centers, gabapentin should be phased out over a period of one week, but the exact schedule will vary depending on the specific patient situation. Slower tapers are reported to allow for safer discontinuation, and it is recommended to reduce the daily dose at a maximum rate of 300 mg every 4 days. The abuse of gabapentin and reclassification do not appear to be a local phenomenon based on the United Kingdom recently reclassifying gabapentin as a Schedule III controlled drug in April 2019. 27 The U.K. classification system is similar to the U.S. system in that there are 5 schedules, but it is slightly different in that the scheduling Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. A gabapentin taper chart can provide structure, helping you gradually reduce your dose while minimizing the discomfort that can come with stopping too quickly. It’s not about rushing—it’s about finding a steady, safe way forward that works for you. Why Not Stop Gabapentin All at Once? Effective July 1, 2018, all gabapentin products became Schedule V controlled substances in the state of Tennessee. Gabapentin is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Pregabalin and gabapentin should only be prescribed where there is evidence of neuropathic changes / neuropathic pain, and even then 50% of patients will not get any benefit at all. Prescribing of gabapentinoids for neuropathic pain should be reviewed in line with the criteria set out in NICE CG173 for neuropathic pain Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The oral bioavailability of gabapentin is approximately 80% at 100 mg administered three times daily once every 8 hours, but decreases to 60% at 300 mg, 47% at 400 mg, 34% at 800 mg, 33% at 1,200 mg, and 27% at 1,600 mg, all with the same dosing schedule. For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum • Gabapentin: Policymakers are increasingly interested in monitoring Gabapentin due to a recent uptick in Gabapentin prescriptions and its regular involvement in overdoses. As a drug that can curb opioid withdrawals and lessen the effects of medications used for addiction treatment, Gabapentin is widely misused. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Our findings indicate that Schedule V changes to gabapentin implemented in three states significantly reduced gabapentin prescribing behavior in Medicare Part D enrollee prescribers. In contrast, we found a modest decrease in gabapentin prescribing for states that implemented PDMP regulation. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. by state and year. States with a gabapentin schedule change or PDMP regulation enacted before 2019 were included in the intervention group. For the Schedule V DID analysis, a control group of the ten highest opioid-prescribing states was used. INTERVENTIONS: States with gabapentin schedule changes or PDMP regulation before January 1, 2019, 2. Is Gabapentin a controlled substance in North Carolina? • No, Gabapentin is not a controlled substance in North Carolina. 3. Why is Gabapentin included in the NC CSRS if it isn’t a controlled substance? • There is evidence that Gabapentin, when taken with opioids, can increase the risk of unintended overdose. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |