Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
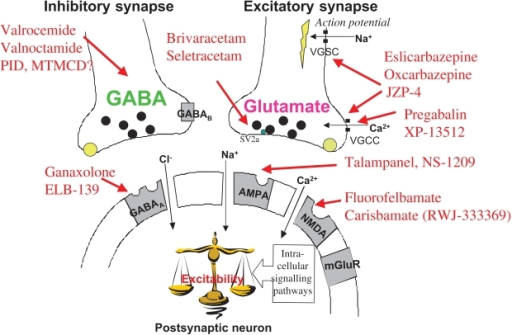
Co-administration of gabapentin with antacids containing aluminium and magnesium, reduces gabapentin bioavailability up to 24%. It is recommended that gabapentin be taken at the earliest two hours following antacid administration. The starting and recommended maintenance dose is 60 mg daily with or without food. Dosages above 60 mg once daily, up to a maximum dose of 120 mg per day administered in evenly divided doses, The EMA guideline states that for drugs with a less than proportional increase in AUC with increasing dose over the therapeutic dose range, bioequivalence should be established both at the highest strength and at the lowest strength (or a strength in the linear range). Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Taking into account the PRAC Assessment Report on the PSUR(s) for gabapentin, the scientific conclusions are as follows: In view of the available data, including post-marketing reports and the literature review, there is sufficient evidence for a causal relation between in-utero exposure to gabapentin and the occurrence of No dose adjustment is required for patients with hepatic impairment (see section 5.2). Paediatric population . The safety and efficacy of Lyrica in children below the age of 12 years and in adolescents (12-17 years established with the start of gabapentin and the onset of agitation, positive de-challenge and positive re-challenge. Therefore the term has been included in section 4.8 of the SmPC. PRAC also reviewed the medical literature and the post marketing safety data regarding the signal of anaphylaxis. Based on this Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). Lyrica is a medicine that contains the active substance pregabalin. It is available as capsules (white: 25, 50 and 150 mg; white and orange: 75, 225 and 300 mg; orange: 100 mg; light orange: 200 mg) and as an oral solution (20 mg/ml). Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is structurally related to the neurotransmitter GABA (gamma-aminobutyric acid) and interacts with GABA synapses. European Medicines Agency Domenico Scarlattilaan 6 1083 HS Amsterdam The Netherlands. Tel: +31 (0)88 781 6000. How to find us Postal address and deliveries The active substance is gabapentin, an established active substance. A draft monograph for gabapentin is published in Pharmeuropa 21.3 dated July 2009. The chemical-pharmaceutical documentation and Expert Report in relation to Gabapentin are of sufficient quality in view of the present European regulatory requirements Manufacturing process Gabapentin A9451182 NON-INTERVENTIONAL FINAL STUDY REPORT 08September2022 PFIZER CONFIDENTIAL Page 2of 91 NON-INTERVENTIONAL (NI) FINAL STUDY REPORT PASS information Title A Population-based Study of the Safety of Gabapentin Use During Pregnancy Protocol number A9451182 Version identifierof the final study report 1.0 Date 08 September 2022 Gabapentin AAA® 400 mg Hartkapseln 2. QUALITATIVE UND QUANTITATIVE ZUSAMMENSETZUNG Jede 100 mg Hartkapsel enthält 100 mg Gabapentin. Jede 300 mg Hartkapsel enthält 300 mg Gabapentin. Jede 400 mg Hartkapsel enthält 400 mg Gabapentin. Sonstiger Bestandteil mit bekannter Wirkung: Jede 100 mg Hartkapsel enthält 22,5 mg Lactose. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). Gabapentin “Pfizer” 300 mg Capsules, hard Oral use Pfizer Aps. Lautrupvang 8 2750 Ballerup Gabapentin “Pfizer” 400 mg Capsules, hard Oral use Pfizer Aps. Lautrupvang 8 2750 Ballerup Gabapentin “Pfizer” 600 mg Film-coated tablets Oral use Pfizer Aps. Lautrupvang 8 2750 Ballerup Gabapentin “Pfizer” Description of update: Type IB (C.I.2.a) variation application to update SmPC and PIL information in-line with the product information of reference product (Neurontin Hard capsule; EU reference No: DE/H/0899/001; MAH: Upjohn EESV) for Gabapentin Accord 100/300/400 mg hard capsules. PIL sections updated: 4, 6. No changes to SmPC. The current study aims to evaluate the effects of gabapentin use in pregnancy on outcomes including malformations, foetal growth indicators, and neurologic morbidity in a large population-based setting.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |