Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
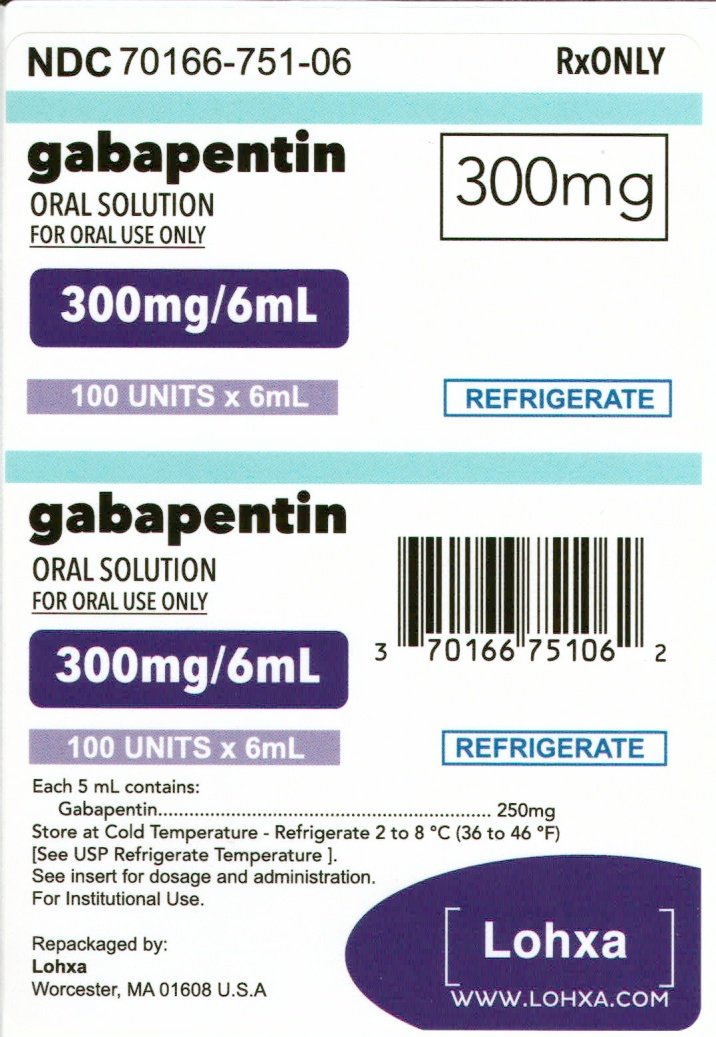 |  |
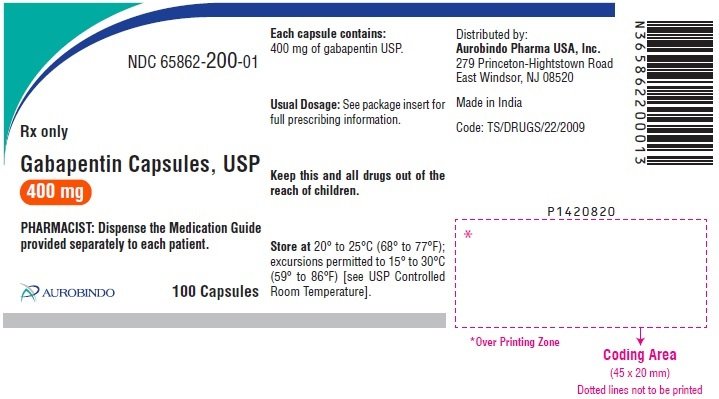
Gabapentin Oral Solution is indicated for: • Management of postherpetic neuralgia in adults • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of somnolence caused by NEURONTIN, can be imperfect. The duration of . GABAPENTIN- gabapentin solution Advagen Pharma Ltd-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral capsule. Stopping gabapentin oral solution suddenly can cause serious problems. Gabapentin oral solution can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin oral solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Gabapentin Oral Solution is supplied as follows: 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available Advise the patient to read the FDA-approved patient labeling (Medication Guide). Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. 2.1 Dosage for Postherpetic Neuralgia . In adults with postherpetic neuralgia, gabapentin oral solution may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary What is Gabapentin Oral Solution? z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. z Partial seizures when taken together with other medicines in adults and children 3 years of age and older. Who should not take Gabapentin Oral Solution? 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or talking with your healthcare provider. Taking gabapentin with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse. • Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin affects you. Gabapentin can slow your thinking and motor skills. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of The recommended maintenance dose of gabapentin capsules in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use Gabapentin oral solution
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |