Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
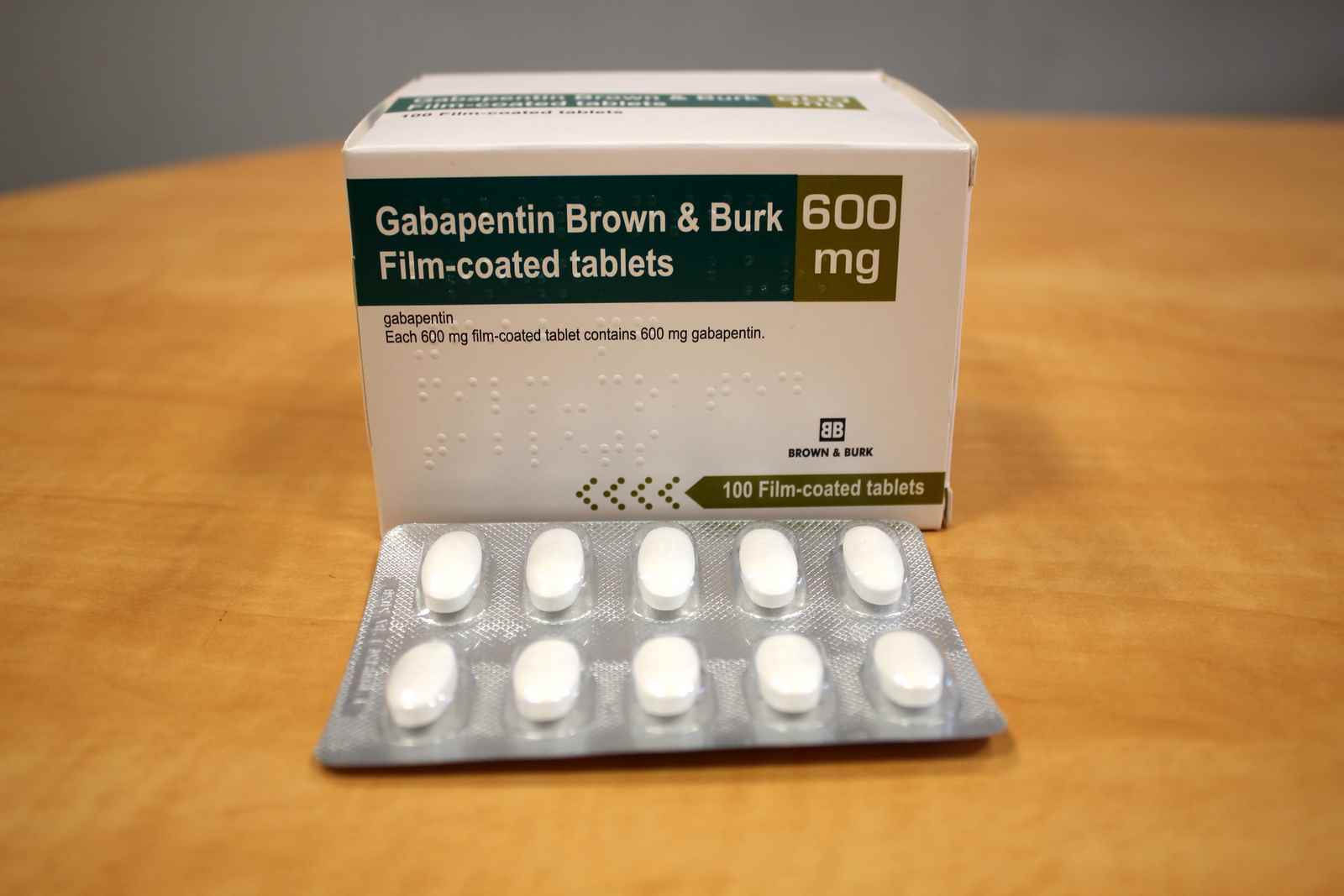
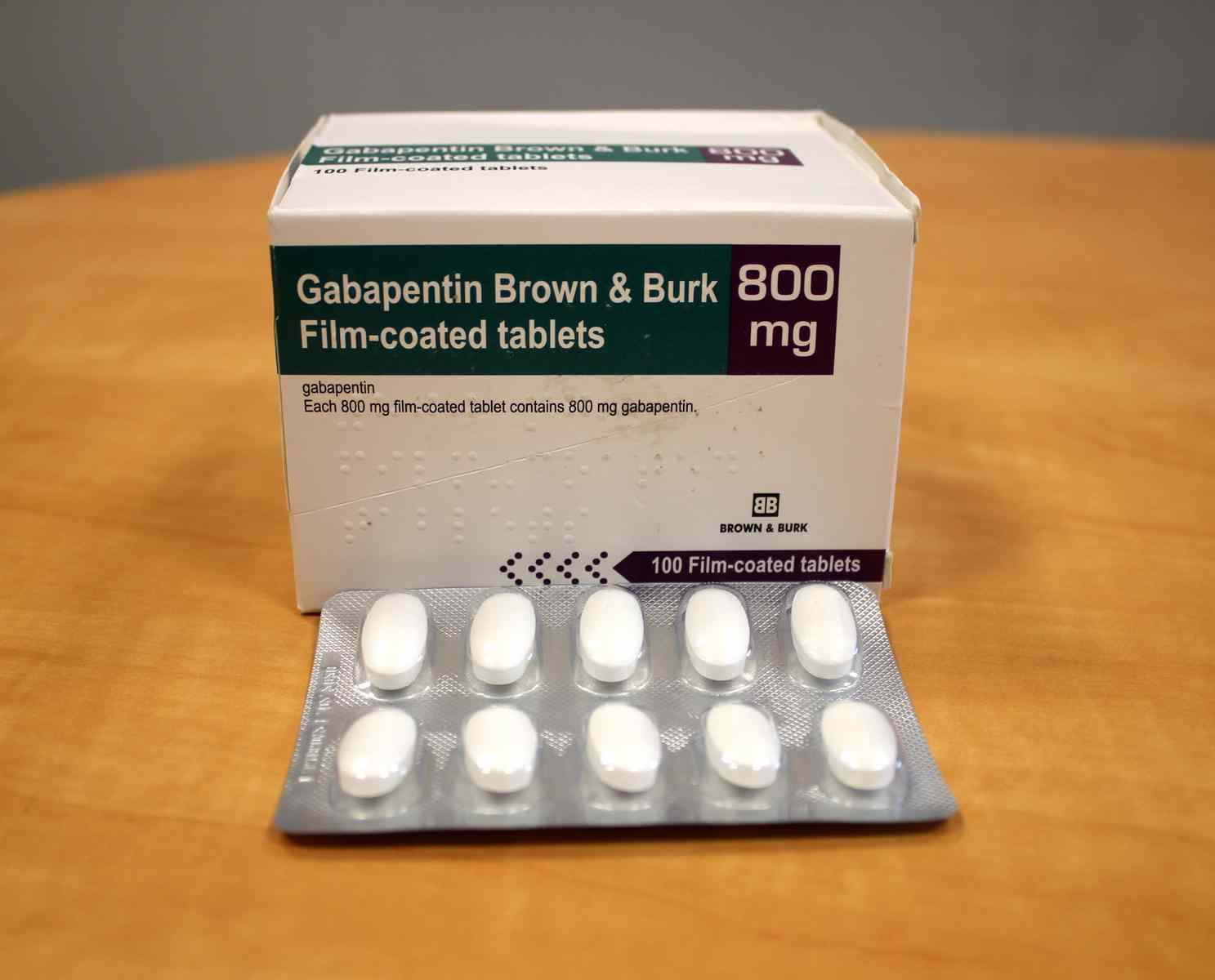
The medication received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly TEVA-GABAPENTIN (gabapentin) Product Monograph Page 7 of 32 administration was revealed in reproduction studies in mice at doses up to 62 times, and in rats and rabbits at doses up to 31 times the human dose of 2400 mg/day. Different brands of gabapentin are not interchangeable and they are FDA approved for different conditions. Use only the brand and form of gabapentin your doctor has prescribed. Check your medicine each time you get a refill to make sure you receive the correct form. Gabapentin tablets are indicated for: In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Gabapentin and pregabalin are FDA-approved for a variety of uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use Initial U.S. Approval: 2011 Pharmaceuticals, LLC at 1-866-516-4950 or FDA at 1-800-FDA-1088 . Gabapentin. is most commonly used in veterinary medicine to relieve chronic. pain and in some pets, to reduce fear and anxiety associated with veterinary. appointments. Gabapentin may be used alone or in combination with other drugs. Gabapentin is available as capsules, tablets and as an oral solution. GENERAL DESCRIPTION: Gabapentin An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate release by about gabapentin, gabapentin sucralfate, sucralfate abraxis pharm * abraxis pharmaceutical products clindamycin phosphate in dextrose 5%, clindamycin phosphate acacia * acacia pharma ltd This label may not be the latest approved by FDA. For current labeling information, please visit particular, gabapentin prevents pain-related responses in NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Precautions, Respiratory Depression (5.7) 4/2020 The Center for Drug Evaluation and Research (CDER) ensures that safe and effective drugs are available to improve the health of the people in the United States Gabapentin caused a marked decrease in neuronal synapse formation in brains of intact mice and abnormal neuronal synapse formation in a mouse model of synaptic repair. Gabapentin has been shown in vitro to interfere with activity of the α2δ subunit of voltage-activated calcium channels, a receptor involved in neuronal synaptogenesis. The
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |