Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
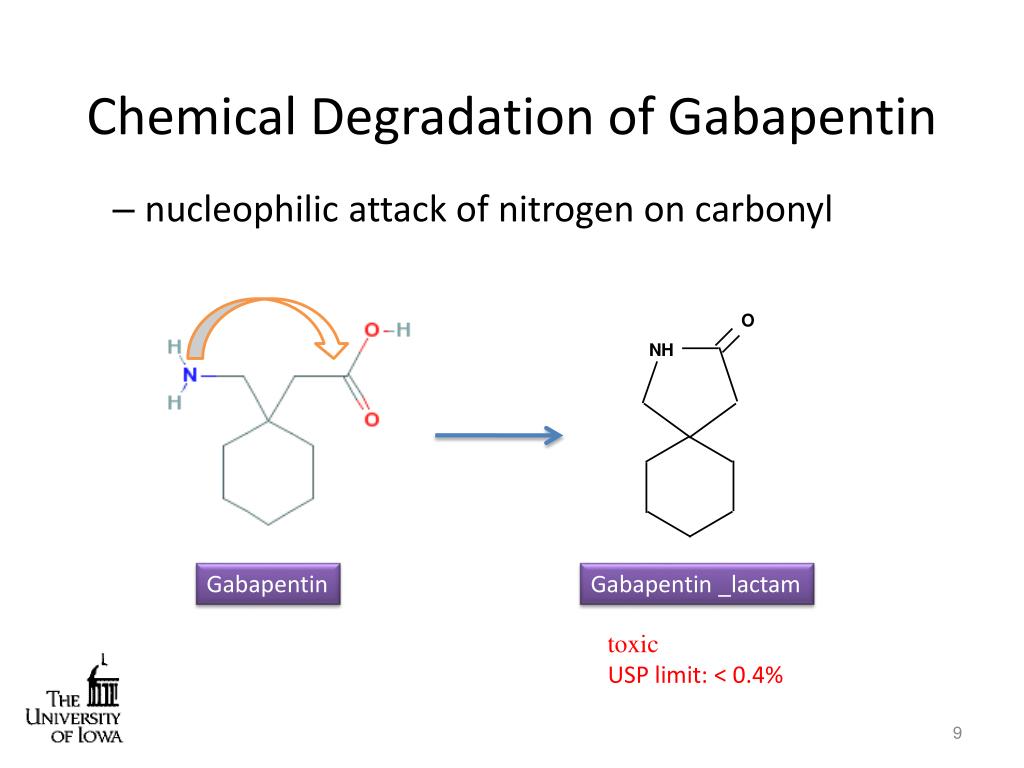
In this paper, we focused on the process intensification of Gabapentin synthesis using a microreaction system. Meanwhile, we systematically studied the effects of concentration and reaction temperature on the reaction rate. In this paper, we focused on the process intensification of Gabapentin synthesis using a microreaction system. Meanwhile, we systematically studied the effects of concentration and reaction temperature on the reaction rate. Therefore, the present invention provides a process for 1,1-cyclohexane diacetic acid monoamide, useful in the synthesis of gabapentin and an improved process for gabapentin, wherein the improvement comprises a new process for isolation of Form II polymorphic form of gabapentin. An efficient chemoenzymatic process is devised for synthesizing high-purity gabapentin. 1-Cyanocyclohexaneacetic acid was first produced in 0.94 M from 1.0 M 1-cyanocycloalkaneacetonitrile by a greatly improved nitrilase from Acidovorax facilis ZJB09122, resulting in a commercially attractive bioprocess with an outstanding space-time yield of 461 g/L/day. The whole cells expressing engineered nitrilase were immobilized in diatomite cross-linked with polyethyleneimine and glutaraldehyde to improve their reusability and stability for the synthesis of gabapentin intermediate 1-cyanocyclohexaneacetic acid from 1-cyanocyclohexaneacetonitrile. The present invention relates to an improved process for the preparation of Gabapentin. The process also relates to a new process for the preparation of 1, 1-cyclohexane diacetic acid monoamide Gabapentin resulted from the search for synthetic analogues of γ-aminobutyric acid (GABA), a natural inhibitor of neurotransmission in the mammalian brain. This chapter illustrates how research carried out in the pharmaceutical industry towards the production of the active pharmaceutical ingredients and worldwide sales of the finished drug Synthesis of gabapentin. A process for chemical synthesis and isolation of gabapentin with high yield and purity [107] starts with conversion of 1,1-cyclohexanediacetic anhydride to 1,1-cyclohexanediacetic acid monoamide and is followed by a 'Hofmann' rearrangement in an aqueous solution of sodium hypobromite prepared in situ. This study deals with insilico docking analysis of gabapentin, phosphatidylcholine, and their conjugate to target Phospholipase A2 enzyme followed by formulation and evaluation of phosphatidylcholine This impurity has been identified, synthesized, isolated and characterized using modern analytical tool. The novel impurity was confirmed as adduct of Gabapentin and lactose; an excipient used in formulation. The elucidated impurity was further confirmed by its synthesis, which was formed due to Maillard reaction and Amadori rearrangement. The present patent application discloses a method for the preparation of [(1-aminomethyl)cyclohexyl]acetic acid (Gabapentin) by means of the Hofmann re-arrangement reaction and the extraction of the Gabapentin from the reaction mixture with a higher aliphatic alcohol, wherein the Hofmann reaction is conducted continuously, for the purpose of obtaining an active ingredient with a good yield and the present invention provides a process for 1,1-cyclohexane diacetic acid monoamide, useful in the synthesis of gabapentin and an improved process for gabapentin, wherein the the preparation of gabapentin of formula (I) or gabalactam of formula (VII) or mixture of gabapentin and gabalactam comprises catalytic hydrogenation of compound (VI) under elevated temperature US5086413 discloses the hydrolysis of diethyl-1-cyanocyclohexyl malonate to 1-cyanocyclohexyl malonic acid followed by decarboxylation and reduction to gabapentin. US5091567 discloses the synthesis of gabapentin in a five step process comprising reaction of cyclohexanone with triethyl phosphonoacetic acid and a base to produce cyclohexylidene synthesis of 1,1-cyclohexane diacetic acid monoamide, inter mediate for the synthesis of gabapentin which ameliorates most of the problems associated with known procedures and is easy to translate to industrial production. 0014. Therefore, the present invention provides a process for 1,1-cyclohexane diacetic acid monoamide, useful in the A novel process has been described on 100 g scale for the preparation of gabapentin lactam which is a penultimate intermediate for the preparation of gabapentin, comprising a Hofmann reaction of 1,1-cyclohexanediacetic acid monoamide using chlorinating agents such as trichloroisocyanuric acid, sodium dichloroisocyanurate, 1,3-dichloro-5,5 We would like to show you a description here but the site won’t allow us. a specific method for gabapentin synthesis given in U.S. Pat. Nos. 4,024,175 and 4,087,544 is as follows (see Scheme I): monomethyl ester of cyclohexane-1,1-diacetic acid was transformed to the corresponding azide which was decomposed (Curtius reaction) in boiling toluene. The resultant isocyanate was hydrolyzed with aqueous hydrochloric acid. A process for preparation of gabapentin comprising a step of obtaining 1,1-cyclohexane diacetic acid monoamide from 1,1-cyclohexane diacetic acid anhydride, wherein said reaction is characterized by the use of ammonia precursor or pre-generated ammonia-isopropanol solution. A method for synthesizing gabapentin hydrochloride, the method uses 1,1-cyclohexyloxalic acid monoamide as the initial raw material, and prepares the target product through the process steps of Hofmann degradation reaction, dehydration condensation, product phase transfer and hydrolysis .
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |