Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
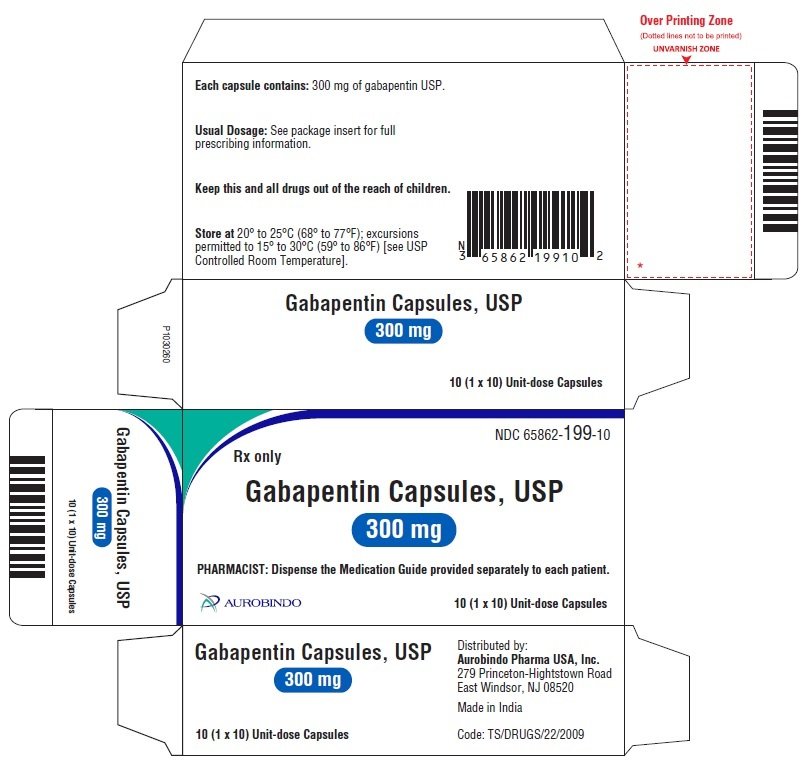 |  |
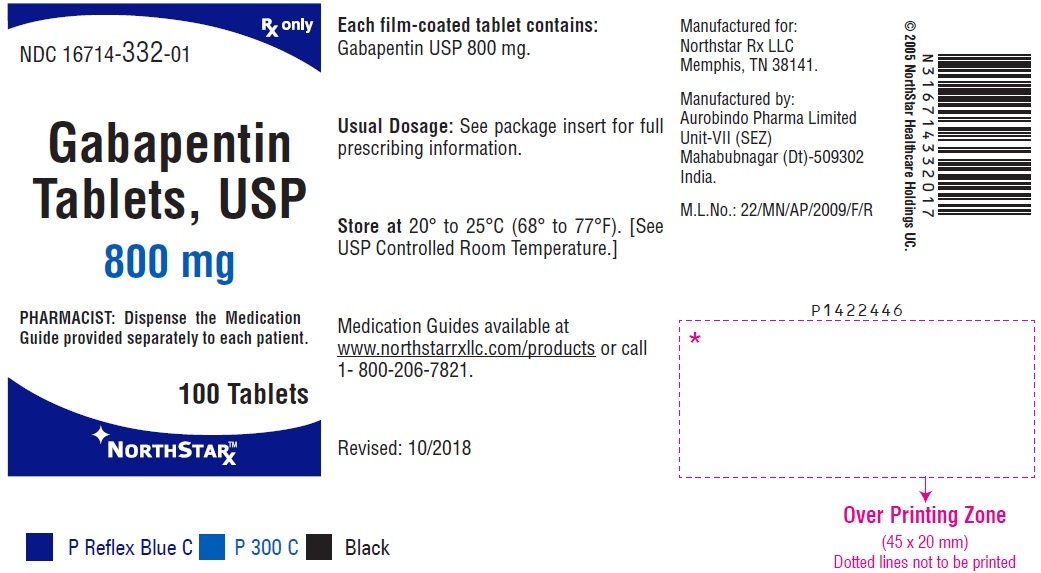
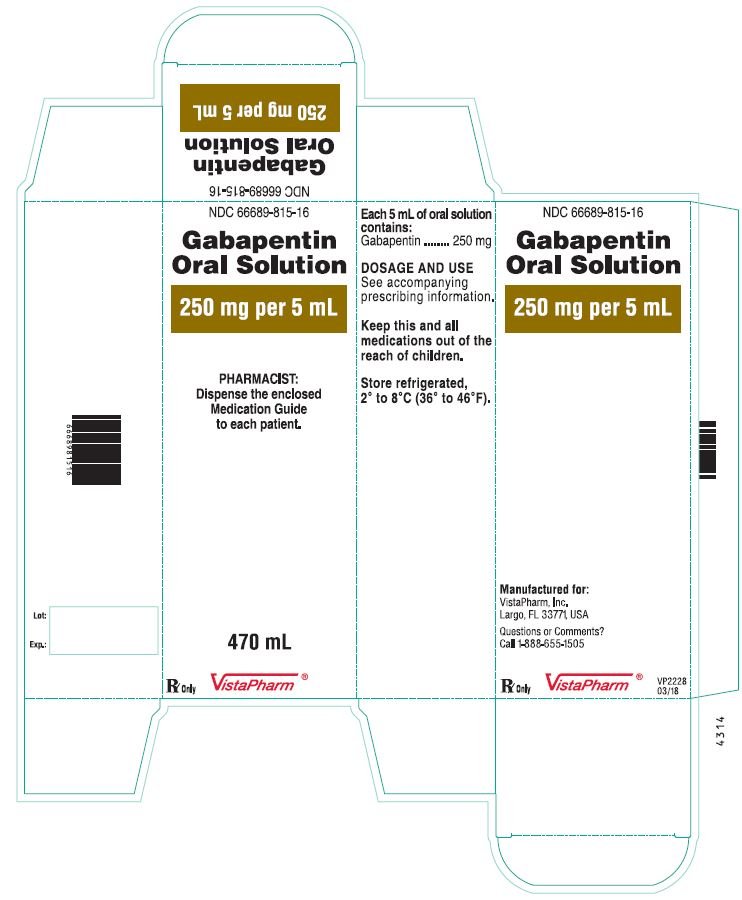
Gabapentin Tablets package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. IN TABLETS. GABAPENTIN tablets, for oral use Initial U.S. Ap. ided doses. The recommended dose is reached by upward titration over a period of approxim. three t. Dosage and Use: See package insert . for full prescribing information. T hese highlights do not include all the information needed to use gabapentin capsules 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. NEURONTIN capsules should be swallowed whole with water. Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Reference ID: 4168942 GABAPENTIN- gabapentin capsule Cipla USA Inc.-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE 2.1 Dosage for Postherpetic Neuralgia. In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Administer gabapentin three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of Administer gabapentin capsules orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin capsules dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. GABAPENTIN- gabapentin tablet, film coated GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. NEURONTIN capsules should be swallowed whole with water. Inform patients that, shouldthey dividethe scored 600mgor 800mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividingthe scored tablet should be discarded. Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence 2.3 Dosage Adjustment in Patients with Renal Impairment Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication): TABLE 1. Gabapentin Capsules Dosage Based on Renal Function Renal Function Creatinine NEURONTIN (gabapentin) capsules, tablets, and oral solution are supplied as follows: 100 mg capsules: White hard gelatin capsules printed with "PD" on the body and "Neurontin/100 mg" on the cap; available in: Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |