Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
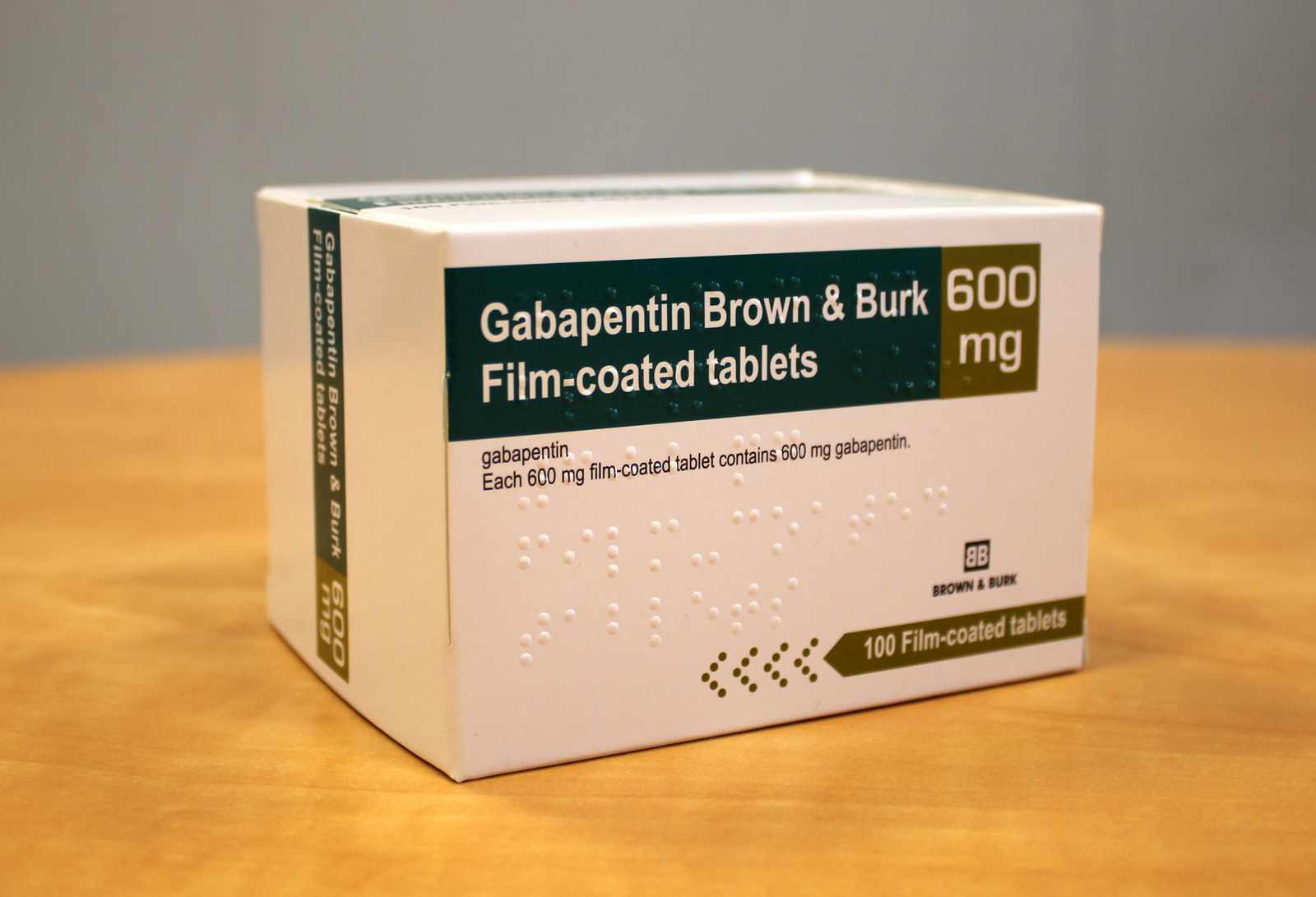
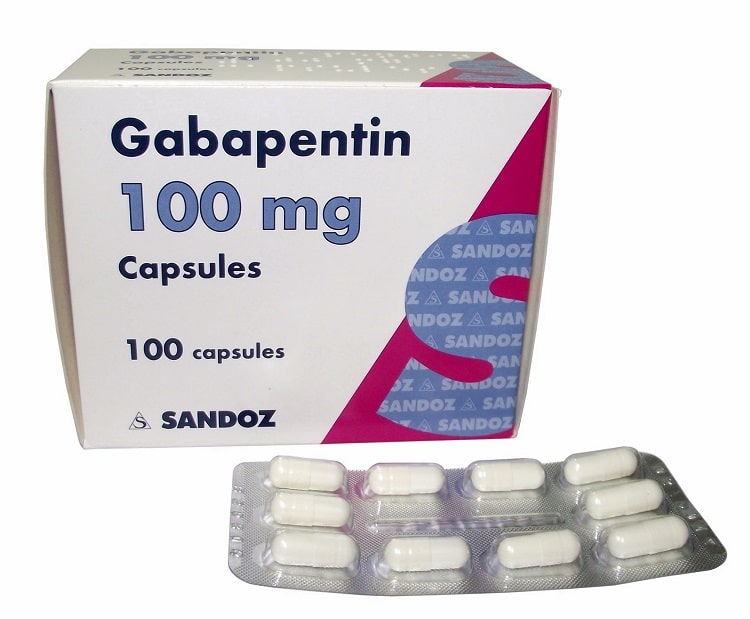
Horizant (gabapentin enacarbil): Take orally once a day about 5 PM for Restless Leg Syndrome or twice a day in the morning and evening for Postherpetic Neuralgia; swallow tablets whole with food; do not cut, crush, or chew tablets; Gralise extended-release tablets should be taken once daily with the evening meal; do not cut, crush, or chew tablets Film-coated tablet. Gabapentin 600 mg film-coated tablets: White to off white coloured, film coated, elliptical shaped, lip break line on both sides, debossed with "S" and "l" separated by lip break line on one side. Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Gabapentin may have minor or moderate influence on the ability to drive and use machines. Gabapentin acts on the central nervous system and may cause drowsiness, dizziness or other related symptoms. Even, if they were only of mild or moderate degree, these undesirable effects could be potentially dangerous in patients driving or operating Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. 3 days. The recommended maintenance Gabapentin can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. How can I watch for early symptoms of suicidal thoughts and actions? GABAPENTIN tablets, for oral use - Initial Gabapentin is indicated for: • Management of postherpetic neuralgia in adults - • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults Gabapentin readily enters the brain and prevents seizures in a number of animal models of epilepsy. Gabapentin does not possess affinity for either GABAA or GABAB receptor nor does it alter the metabolism of GABA. It does not bind to other neurotransmitter receptors of the brain and does not interact with sodium channels. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin may have minor or moderate influence on the ability to drive and use machines. Gabapentin acts on the central nervous system and may cause drowsiness, dizziness or other related symptoms. Even, if they were only of mild or moderate degree, these undesirable effects could be potentially dangerous in patients driving or operating Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin, the active substance of Neurontin, is passed on through human milk. Because the effect on the baby is unknown, it is not recommended to breast-feed while using Neurontin. Fertility There is no effect on fertility in animal studies. Driving and using machines Neurontin may produce dizziness, drowsiness and tiredness. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Gabapentin readily enters the brain and prevents seizures in a number of animal models of epilepsy. Gabapentin does not possess affinity for either GABAA or GABAB receptor nor does it alter the metabolism of GABA. It does not bind to other neurotransmitter receptors of the brain and does not interact with sodium channels. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. Gabapentin 600 mg film-coated tablets - Patient Information Leaflet (PIL) by Aurobindo Pharma - Milpharm Ltd. Each film-coated tablet contains 600 mg gabapentin. For the full list of excipients, see section 6.1. Search emc: Enter medicine name or company Gabapentin 300 mg capsules Active Ingredient: gabapentin. Company: Rivopharm UK Ltd See contact details ATC code
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |