Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
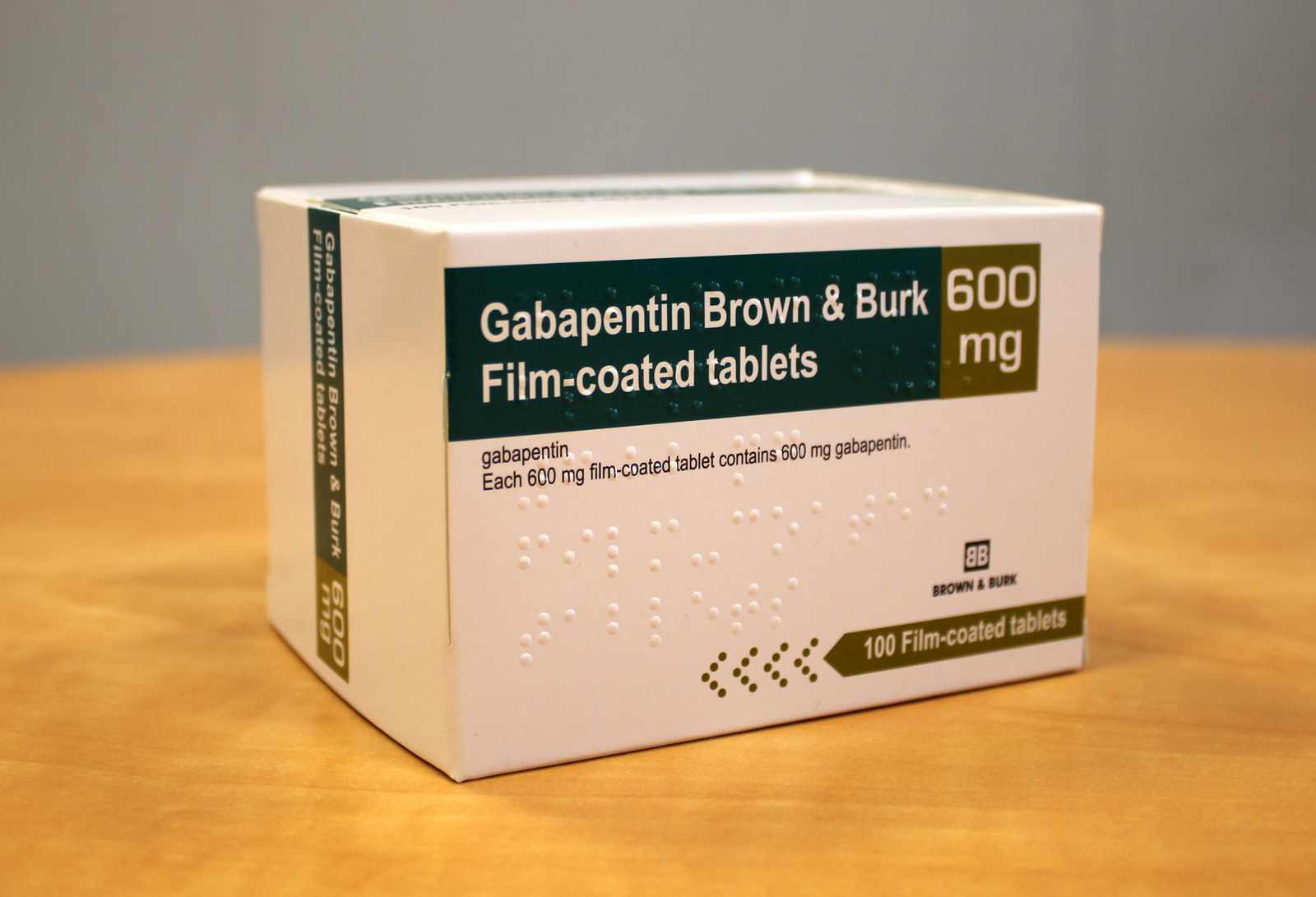
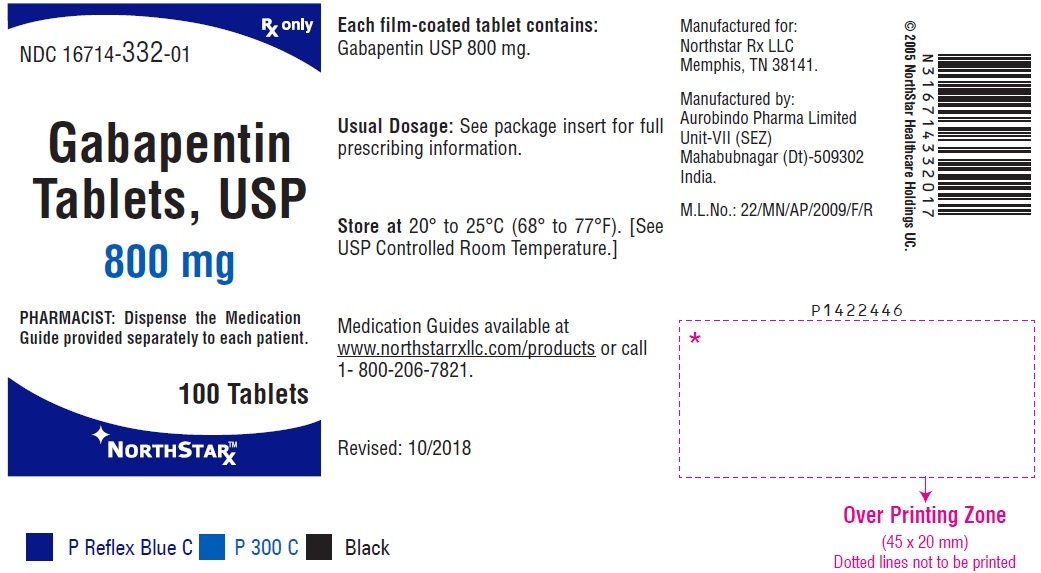
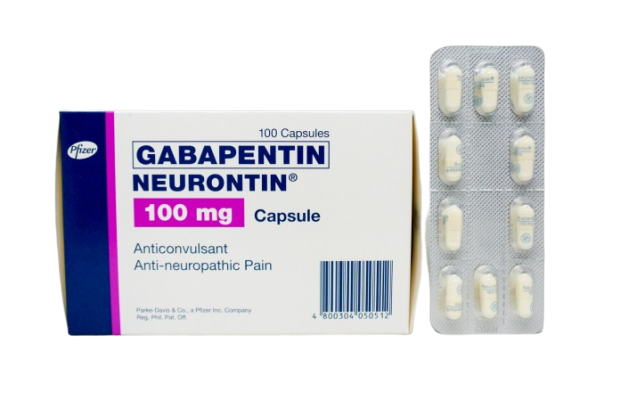
Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc. Gabapentin Tablets USP. Generic Version of Neurontin® Tablets Strength: 600 mg. Available Sizes [NDC & Pack]: 68462-0126-01. 100-Count Bottle. 68462-0126-05. Gabapentin tablets, USP are white colored film coated, modified capsule shaped biconvex tablets containing 600 mg and 800 mg of gabapentin, USP. The inactive ingredients are mannitol, Hydroxypropyl Cellulose, Crospovidone, Talc, Magnesium stearate and aquarius® BP18114 Cool Vanilla. Gabapentin Tablets, USP are indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Tablets labeled to contain 600 or 800mg) S is the concentration, in mg per mL, of USP Gabapentin equivalent to about 100mg of gabapentin, to a suitable volumetric GABAPENTIN - gabapentin tablet, film coated Sun Pharmaceutical Industries Limited -----Gabapentin Tablets, USP DESCRIPTION Gabapentin tablets, USP are supplied as elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are glyceryl behenate, hydroxypropyl cellulose, low substituted Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Each gabapentin tablet, USP intended for oral administration contains 600 mg and 800 mg of gabapentin. In addition each tablet contains following inactive ingredients: copovidone, low substituted hydroxypropyl cellulose, magnesium stearate, mannitol, poloxamer, povidone and talc. In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). Committed to healthier life. We add value through superior customer service in the distribution of a broad line of generic pharmaceuticals, leveraging vertical integration and efficient controlled processes. All Rights Reserved. Strides Pharma Science Limited (Strides) announced that its step‐ down wholly owned subsidiary, Strides Pharma Global Pte. Limited, Singapore, has received approval for Gabapentin Tablets USP, 600 mg and 800 mg, from the United States Food & Drug Administration (USFDA). The product is bioequivalent and therapeutically equivalent to the Reference Listed Drug (RLD) Neurontin Tablets, 600 mg 600 mg NDC 71717-102-50 Gabapentin Tablets, USP Rx only 500 Tablets 220mm 80mm Manufactured by: CSPC Ouyi Pharmaceutical Co., Ltd. Shijiazhuang, Hebei, China, 052160 LOT : EXP Distributed by : : Megalith Pharmaceuticals Inc. Princeton, NJ 08540 Each film-coated tablet contains 600 mg of gabapentin, USP. USUAL DOSAGE See package insert for Gabapentin is a medication that treats nerve pain by calming overactive nerves in your body. It may also prevent and control seizures in people with epilepsy. You can take this medication by mouth with a glass of water. It is used to treat seizures. It is used to treat painful nerve diseases. It may be given to you for other reasons. Talk with the doctor. What do I need to tell my doctor BEFORE I take Neurontin? Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Gabapentin can cause life-threatening breathing problems, especially if you already have a breathing disorder or if you use other medicines that can make you drowsy or slow your breathing. Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT Mylan Pharmaceuticals ULC 85 Advance Road, Etobicoke, ON M8Z 2S6 Submission Control No.: 202563 Date of Revision: February 14, 2017 Each gabapentin tablet, USP contains 600 mg or 800 mg of gabapentin, USP and the following inactive ingredients: Corn starch, copovidone, poloxamer, and magnesium stearate. The film coating Opadry White contains hydroxypropyl cellulose and talc. FDA approved dissolution test specifications differ from USP. The recommended maintenance dose of gabapentin is 300 mg to 600 mg three times a day. Dosages up to 2,400 mg/day have been administered in long-term clinical studies. Doses of 3,600 mg/day have also been administered to a small number of patients for a relatively short duration. Administer gabapentin three times a day using 600 mg or 800 mg Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. Gabapentin tablets are supplied as oval-shaped film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients are copovidone, hydroxyl propyl cellulose, magnesium stearate mannitol, poloxamer, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |