Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 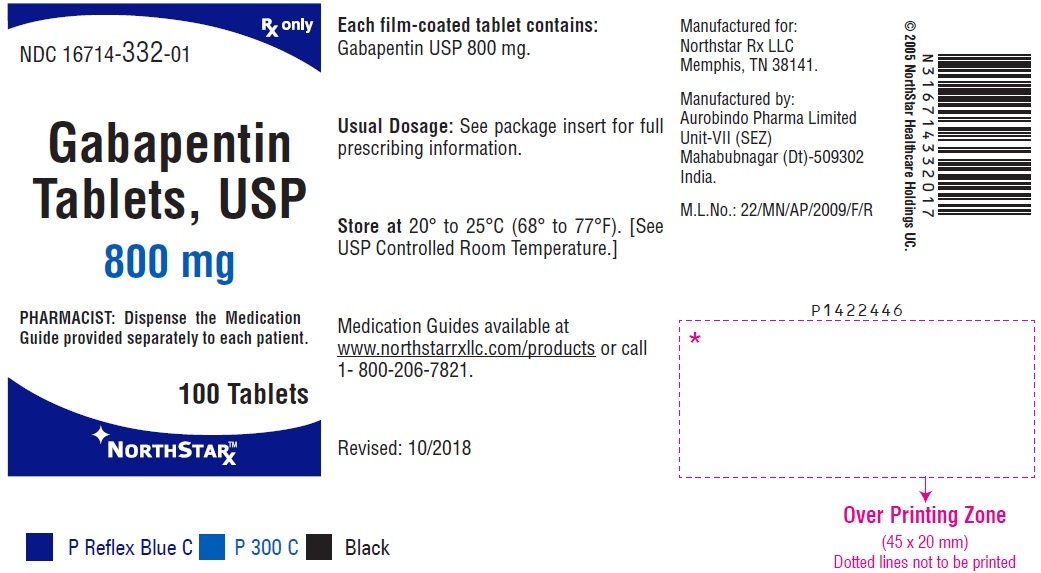 |
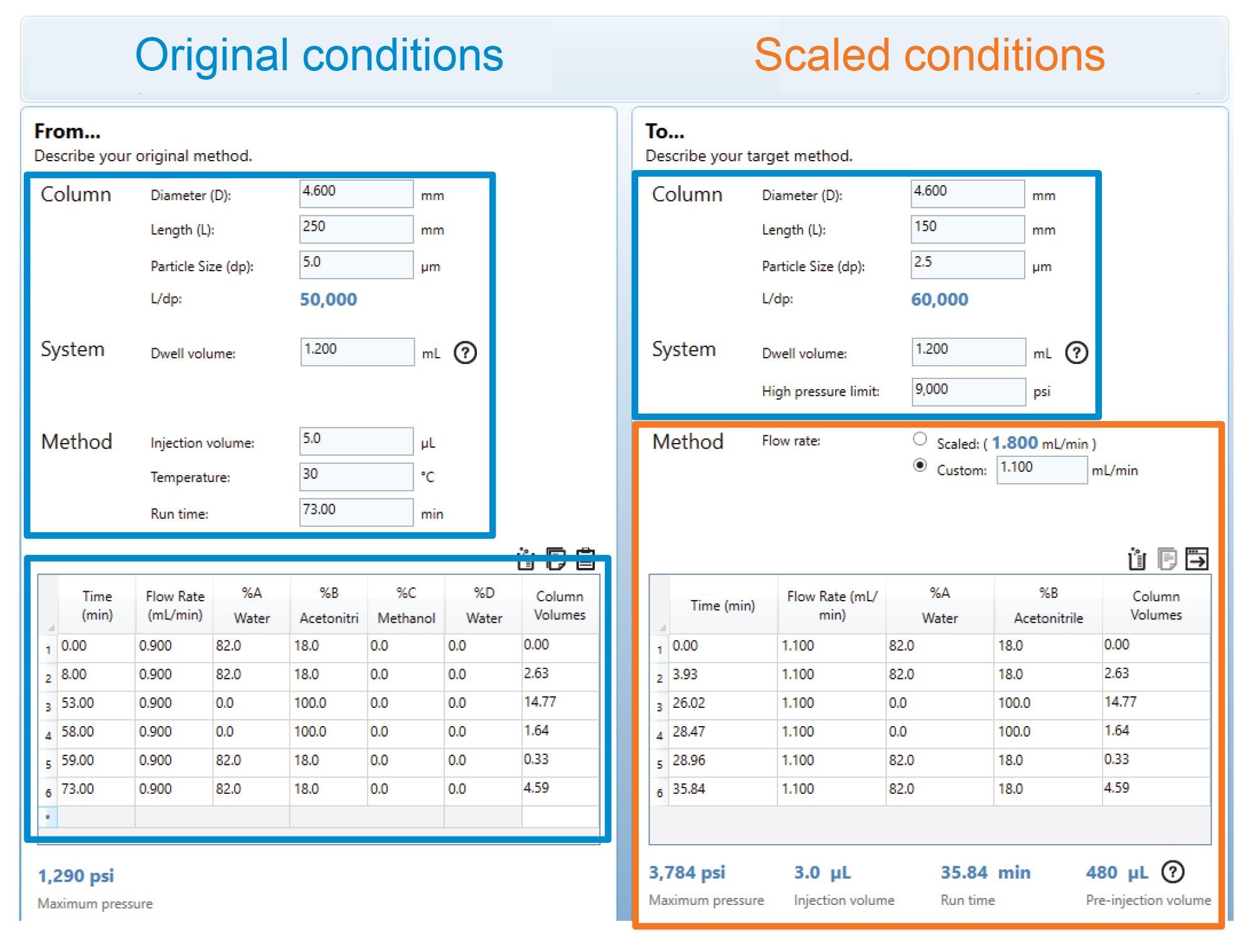
GABAPENTIN TABLETS PF 34(4) Pg. 934 Labeling, Dissolution <711> Margareth Marques GLIMEPIRIDE TABLETS PF 33(3) Pg. 411 Dissolution <711> Margareth Marques GLUTAMIC ACID PF 34(4) Pg. 997 Title, Chemical Info, Definition, Packaging and storage, USP Reference standards <11>, Identification, Specific rotation <781S>, Loss GLN-Gabapentin (Gabapentin Tablets USP, 600 mg and 800 mg) Page 1 of 36 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrGLN-Gabapentin Gabapentin Tablets Tablets, 600 mg and 800 mg, oral USP Antiepileptic Agent Glenmark Pharmaceuticals Canada Inc. 1600 Steeles Ave. West, Suite 407 Concord, Ontario L4K 4M2 In accordance with the Rules and Procedures of the 2005–2010 Council of Experts, the Biopharmaceutics Expert Committee has revised the Tolerances in Dissolution Test 2 as requested by the Food and Drug Administration. The Gabapentin Tablets Revision Bulletin supersedes the monograph in USP 32–NF 27 until it is printed in the USP 33–NF 28 Tablets labeled to contain 600 or 800mg) of the appropriate Work-tin related compound A is not more than 5.0%. ing standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. GABAPENTIN - gabapentin tablet, film coated Sun Pharmaceutical Industries Limited -----Gabapentin Tablets, USP DESCRIPTION Gabapentin tablets, USP are supplied as elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are glyceryl behenate, hydroxypropyl cellulose, low substituted APO-GABAPENTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND PRECAUTIONS, Special Populations). Pro -Gabapentin (Gabapentin Tablets USP, 600 mg and 800 mg) Page 4 of 36 PART I: HEALTH PROFESSIONAL INFORMATION 1 INDICATIONS Adults Pro -Gabapentin (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. 1.1 Pediatrics Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. USP Gabapentin Related Compound A RS . A: Infrared Absorption 197K. Test specimen— Grind at least 20 Tablets to a fine powder. Use an amount of powder, equivalent to about 2 mg of gabapentin, and about 200 mg of dry potassium bromide. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Standard preparation— Dissolve an accurately weighed quantity of USP Gabapentin RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 4.0 mg per mL. Gabapentin Capsules USP and Gabapentin Tablets USP dose is reduced, discontinued or substituted with an alternate anticonvulsant or an alternate anticonvulsant is added to Gabapentin Capsules USP and Gabapentin Tablets USP therapy, this should be done gradually over a minimum of 1 week (a longer Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. United States Pharmacopeia (2024). USP Monographs, Gabapentin. USP-NF. Rockville, MD: United States Pharmacopeia. Gabapentin Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Tablets. USP-NF. Rockville, MD: United States Pharmacopeia. System suitability solution—Dissolve a suitable quantity of USP Gabapentin RS in Diluent, and add an appropriate volume of Impurities solution to obtain a solution containing about 14.0 mg per mL, 0.014 mg per mL, and 0.0084 mg per mL of USP. PRODUCT MONOGRAPH Pr MYLAN-GABAPENTIN (Gabapentin Capsules) 100 mg, 300 mg, and 400 mg (Gabapentin Tablets, USP) 600 mg and 800 mg ANTIEPILEPTIC AGENT Mylan Pharmaceuticals ULC 85 Advance Road, Etobicoke, ON M8Z 2S6 Submission Control No.: 202563 Date of Revision: February 14, 2017 PRODUCT MONOGRAPH PrTEVA-GABAPENTIN Gabapentin Capsules 100 mg, 300 mg, and 400 mg Tablets 600 mg and 800 mg Antiepileptic Agent Teva Standard Teva Canada Limited Table 2. Dosage Adjustments for Renal Impairment in Adults Receiving Gabapentin Gastroretentive Tablets60; Cl cr (mL/minute). Adjusted Dosage Regimen. 30–60. 600 mg to 1.8 g once daily; initiate at 300 mg once daily and may titrate according to same schedule recommended for those with normal renal function based on individual patient response and tolerability Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). United States Pharmacopeia (2024). USP Monographs, Gabapentin Capsules. USP-NF. Rockville, MD: United States Pharmacopeia. Buy [Gabapentin (250 mg)] - CAS [60142-96-3] from USP. * Certain Material Origins (i.e. Animal, Plant, Fish) may require special country importation requirements.USP recommends you contact your country competent authorities to determine if any certifications, permits or licenses may be required prior to ordering.Material Origins are found within the Product under Origin Information.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |