Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 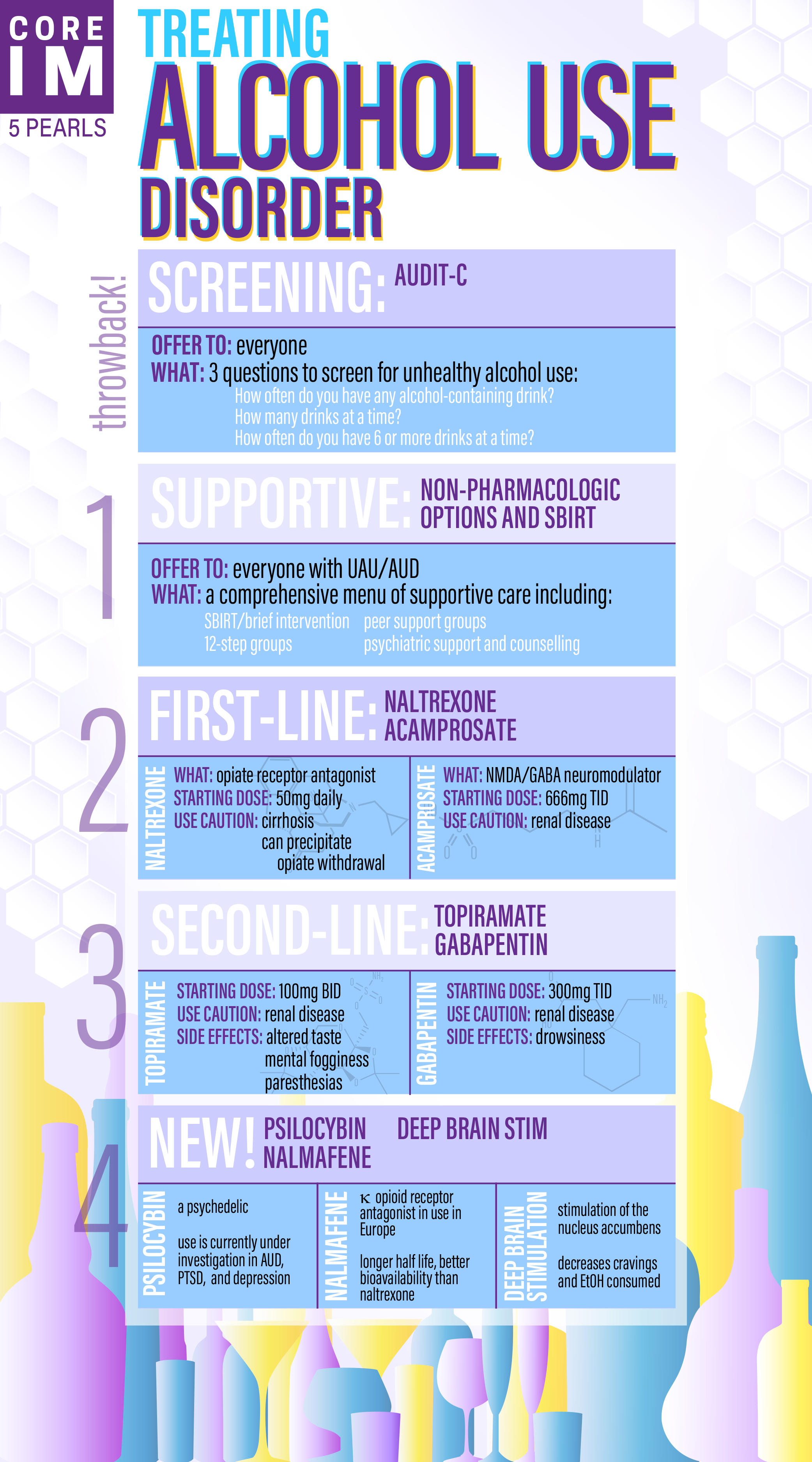 |
Gabapentin may be helpful in treating alcohol use disorder and withdrawal. Between 2004 and 2010, The Veterans Affairs Department conducted a double-blind, placebo-controlled, randomized dose Gabapentin, a prescription medication approved for the treatment of seizures and neuralgia, is often prescribed off-label for substance use treatment, mental health problems, and pain. Emerging reports also suggest it is misused for the purpose of getting high. Gabapentin is used to control seizures, to treat nerve pain that can happen after having had shingles, and to treat a condition called restless legs syndrome. In addition to these FDA-approved uses, doctors sometimes prescribe gabapentin off-label. Gabapentin is effective for the treatment of alcohol dependence and can be used for treating anxiety, insomnia, headaches, and/or pain in patients who have comorbid substance use disorders (SUDs) or who are at high risk of substance abuse. In the USA, ~2.1 million individuals have been diagnosed with opioid use disorder defined as abuse with illicit and/or prescription medications, 1 thereby resulting in significant morbidity, mortality, and an increase in state and federal health service expenditures. 2 In light of these events, federal and state regulatory agencies and Prescriptions for the anticonvulsant gabapentin have been steadily rising for more than a decade, primarily due to off-label use of the medication for a variety of conditions including pain, substance use disorder, and more. In 2021, gabapentin was ranked among the top 10 most prescribed medications in the United States. Evidence does not support the use of gabapentin for bipolar disorder, major depressive disorder (MDD), posttraumatic stress disorder (PTSD), obsessive compulsive disorder (OCD), stimulant use disorder, or opioid withdrawal. Abstract. The aim of the present study was to examine a potential mechanism of action of gabapentin to manage cannabis-use disorders by determining the interoceptive effects of gabapentin in cannabis users discriminating Δ 9-THC using a pharmacologically selective drug-discrimination procedure. Gabapentin was originally approved by the US Food and Drug Administration (FDA) for the treatment of partial seizures in 1993, 1, 2 with subsequent approval for postherpetic neuralgia in 2002. 3 – 5 Within a decade of initial FDA approval, gabapentin’s second most common use became off-label prescription for psychiatric disorders. 6 Cannabis use disorder (CUD) is defined by an inability to stop a problematic pattern of use despite significant negative consequences. CUD has traditionally been thought to affect 1 in 10 people who use cannabis, but recent evidence has indicated that the lifetime incidence may be closer to 1 in 5. 1 As part of this trend in off-label use, gabapentin is frequently prescribed during the treatment of opioid use disorder (OUD) to manage the severity of withdrawal symptoms, or to manage comorbid conditions frequently identified alongside OUD such as mental health issues and chronic pain. 10-13 Individuals with a history of substance use Learn about gabapentin for alcohol use disorder (AUD). Explore how this medication may help with withdrawal symptoms, cravings, and support recovery. Gabapentin: Gabapentin is indicated for postherpetic neuralgia and serves as adjunctive therapy for managing partial seizures (with or without secondary generalization) in adults and pediatric patients aged 3 or older. Off-label, gabapentin is also used to treat mental health problems, including anxiety and post-traumatic stress disorder, as well as in substance use treatment settings for managing withdrawal symptoms (Buttram, Kurtz, Ellis, et al. 2019; Berlin, Butler, and Perloff 2005). Evidence from single-site studies lend support to the safety and efficacy of gabapentin as a novel treatment for alcohol use disorder, with unique benefits for alcohol-related insomnia and negative affect, relative to available treatments. Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms: A Randomized Clinical Trial JAMA Intern Med . 2020 May 1;180(5):728-736. doi: 10.1001/jamainternmed.2020.0249. Keywords: gabapentin, neurontin, bipolar disorder, substance use disorder, alcohol use disorder, alcohol withdrawal, PTSD, anxiety disorder. Introduction. The Food and Drug Administration (FDA) in the United States (US) first licensed gabapentin (GBP) in 1993 as an adjunctive treatment for partial seizures. Gabapentin has been shown to be safe and effective for mild alcohol withdrawal but is not appropriate as mono-therapy for severe withdrawal owing to risk of seizures. During early abstinence, gabapentin may improve sleep, cravings, and mood—factors associated with relapse. Gabapentin is a medication used for epilepsy seizures, restless leg syndrome, and nerve pain caused by shingles. While it has been used to treat other addictions, it’s usually used specifically AUD-Patient AD-Gabapentin Author: US Department of Veterans Affairs Subject: This document focuses on the treatment of alcohol use disorder with Gabapentin. It is a patient education document. Keywords: Academic detailing; Patient education; Alcohol Use Disorder; Pharmacotherapy: Gabapentin Created Date: 5/17/2017 12:39:53 PM
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |