Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
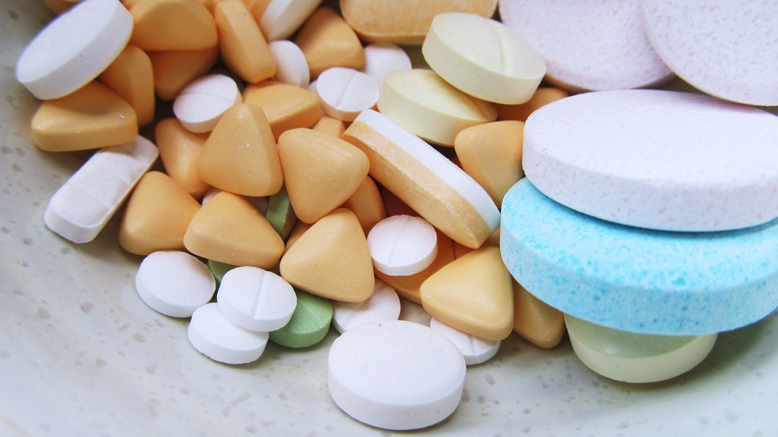
T hese highlights do not include all the information needed to use gabapentin capsules safely and. effectively. See full prescribing information for gabapentin capsules. GABAPENTIN capsules, USP for oral use. Initial U.S. Approval: 1993 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or Sales of Lyrica and Neurontin tripled a decade ago, when they were touted as safer alternatives to opioids and prescribed off-label for a variety of pain conditions. In 2018, Michael Johansen, MD, a researcher and family medicine physician, was one of the first to warn that gabapentinoids were being overprescribed , despite little of evidence The updated FDA pregabalin and gabapentin warnings labels will tell healthcare providers to not prescribe those drugs at the same time as opioids. The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of somnolence caused by NEURONTIN, can be imperfect. The duration of . NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. (5.1) NEURONTIN ® (gabapentin) capsules, for oral use . NEURONTIN ® (gabapentin) tablets, for oral use . NEURONTIN ® (gabapentin) oral solution . Initial U.S. Approval: 1993 ----- INDICATIONS AND USAGE ----- NEURONTIN is indicated for: NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Precautions, Respiratory Depression (5.7) 4/2020 FDA Requires New Warnings on Labels, Clinical Trials into Gabapentinoids. The FDA ordered new warnings of breathing risks on labels for gabapentin and pregabalin in December 2019. The agency is also requiring manufacturers to begin clinical trials to gauge the potential for abuse with the drugs. Known hypersensitivity to gabapentin or its ingredients (4) -----WARNINGS AND PRECAUTIONS----- Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan ypersensitivity): Discontinue Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc. Gabapentin tablets are indicated for: For a complete listing, see 6 DOSAGE FORMS, STRENGTHS, COMPOSITION AND PACKAGING. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. Gabapentinoid products include gabapentin, which is marketed under the name Neurontin and Gralise, as well as generics; gabapentin enacarbil, a prodrug of gabapentin which is marketed as Horizant Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. This text seems to be a label from a medication container for Gabapentin capsules, including information on dosage and the pharmacist's guidance. It indicates details such as the name of the medication, quantity (100 capsules), and NDC code (82619-142.01). The label also notes that this medication is for prescription-only use (Rx Only).* In late December 2019, the U.S. Food and Drug Administration (FDA) announced that it will require new warning labels for gabapentinoids. These labels will address the risk of serious respiratory distress leading to death in patients who combine the treatment with an opioid. This change is based on a review of multiple sources, including case This label may not be the latest approved by FDA. For current labeling information, please visit particular, gabapentin prevents pain-related responses in In vitro studies with radiolabeled gabapentin have revealed a gabapentin binding site in areas of rat brain including neocortex and hippocampus. A high-affinity binding protein in animal brain
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |