Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
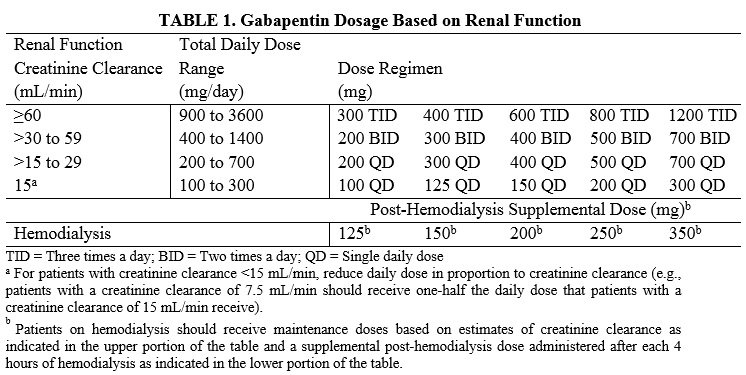 | 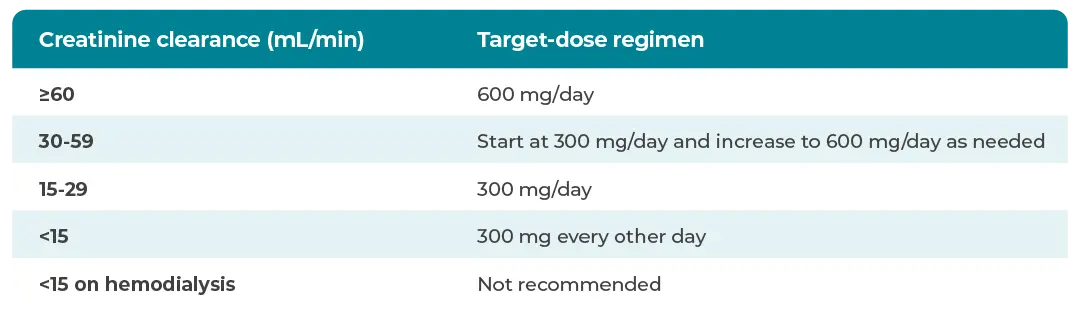 |
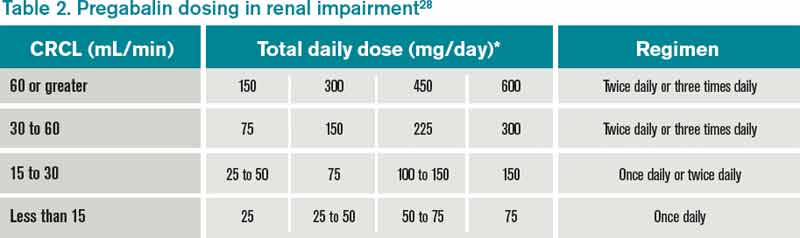
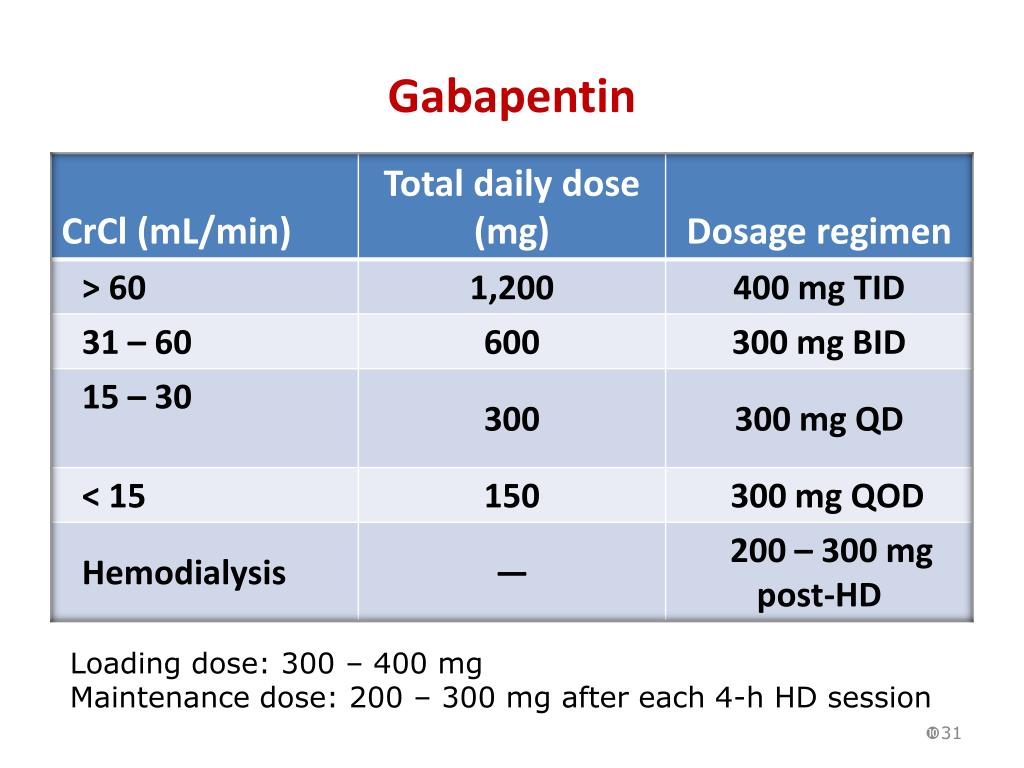
Gabapentin and pregabalin are commonly used for neuropathic pain in CKD patients but are not fully understood as this population remains excluded from efficacy and safety trials. Renal adjustments for the gabapentinoids are prodigiously recommended in the literature. Renal impairment: Gabapentin dose reduction may be required, depending on renal function. Next: Interactions. Interaction Checker. Enter a drug name to check for any Rational dosing of gabapentin and pregabalin in chronic kidney disease normal renal function on maximum recommended dosing yielded concentrations of 5–8 mg/L for gabapentin and ~ 2.8–8.2 mg/L for pregabalin. 22–25 The elimination half-lives of gabapentin and pregabalin are prolonged with renal impairment leading up to accumulation with Gabapentin toxicity in patients with chronic kidney disease is underrecognized. Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. Drug dosing errors are common in patients with renal impairment and can cause adverse effects and poor outcomes. Dosages of drugs cleared renally should be adjusted according to creatinine INTRODUCTION. Pain is one of the most common and distressing symptoms among patients with chronic kidney disease (CKD) [].The prevalence of pain has been associated with substantially lower health-related quality of life and greater psychosocial distress, insomnia, and depressive symptoms []. Gabapentin Dosage Based on Renal Function. For patients with creatinine clearance <15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min receive). Doses often need to be reduced in renal impairment to prevent accumulation and toxicity. Examples of drugs that should be reduced in renal impairment are the gabapentinoids: gabapentin and pregabalin. Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication): Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. View gabapentin information, including dose, uses, side-effects, renal impairment, pregnancy, breast feeding, monitoring requirements and important safety information. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Conclusion. All doctors need to be aware of the need to review the indications for gabapentin use during periods of acute illness, especially with regard to renal impairment. Off-label use should be discouraged. Gabapentin dosing guidelines for adult with renal impairment are summarized in Table 3. Dosing guidelines for gabapentin immediate-release are also applicable for adolescents 12 years of age and older with renal impairment. Gabapentin has also been linked to causing behavioral changes such as confusion and CNS depression including somnolence and dizziness, which is more prominent in patients with renal impairment. (Lexicomp 2016). The Renal Dosage is an educational tool designed by an experienced physician, Dr. Safwan Sayyal in consultation with nephrologists to ascertain the appropriate medication dosage based on a patient's kidney function. This will help to eliminate dosing errors in individuals with renal impairment. Therapeutic dosing targets of both medications have been established in clinical trials for neuropathic pain (gabapentin 1800–3600 mg/day; pregabalin 150–600 mg/day). However, patients with renal impairment were often excluded from these studies. Adjust the dose for people with renal impairment (see Table 2). Consult the manufacturer's Summary of Product Characteristics if the person is undergoing haemodialysis. Table 2. Recommended dosage adjustment for gabapentin in people with renal impairment. Dosage Adjustment in Patients with Renal Impairment . Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication): Reference ID: 4584064 . This label may not be the latest approved by FDA. **Patients with renal impairment are more sensitive to neurological side effects of these drugs and should be carefully monitored** Gabapentin. HD: 100mg after each dialysis session. If required the dose may be titrated in 100mg increments every 7 days to 300mg post HD, according to response and tolerability. PD and CrCl <30mL/min: The risk of respiratory depression should be assessed when initiating gabapentin in patients with respiratory comorbidities, neurological disease, renal impairment and in combination with other respiratory depressants such as opioids.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |