Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 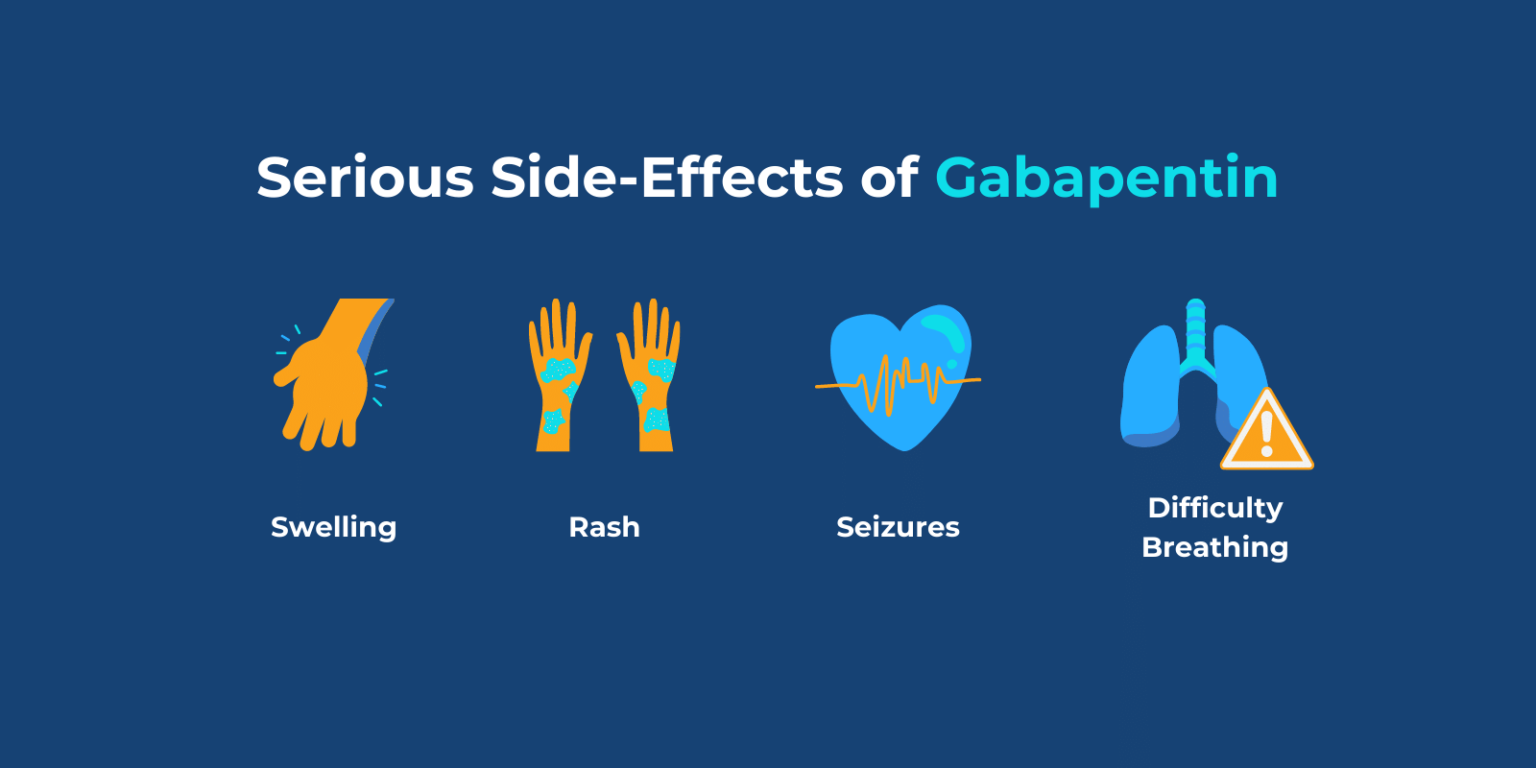 |
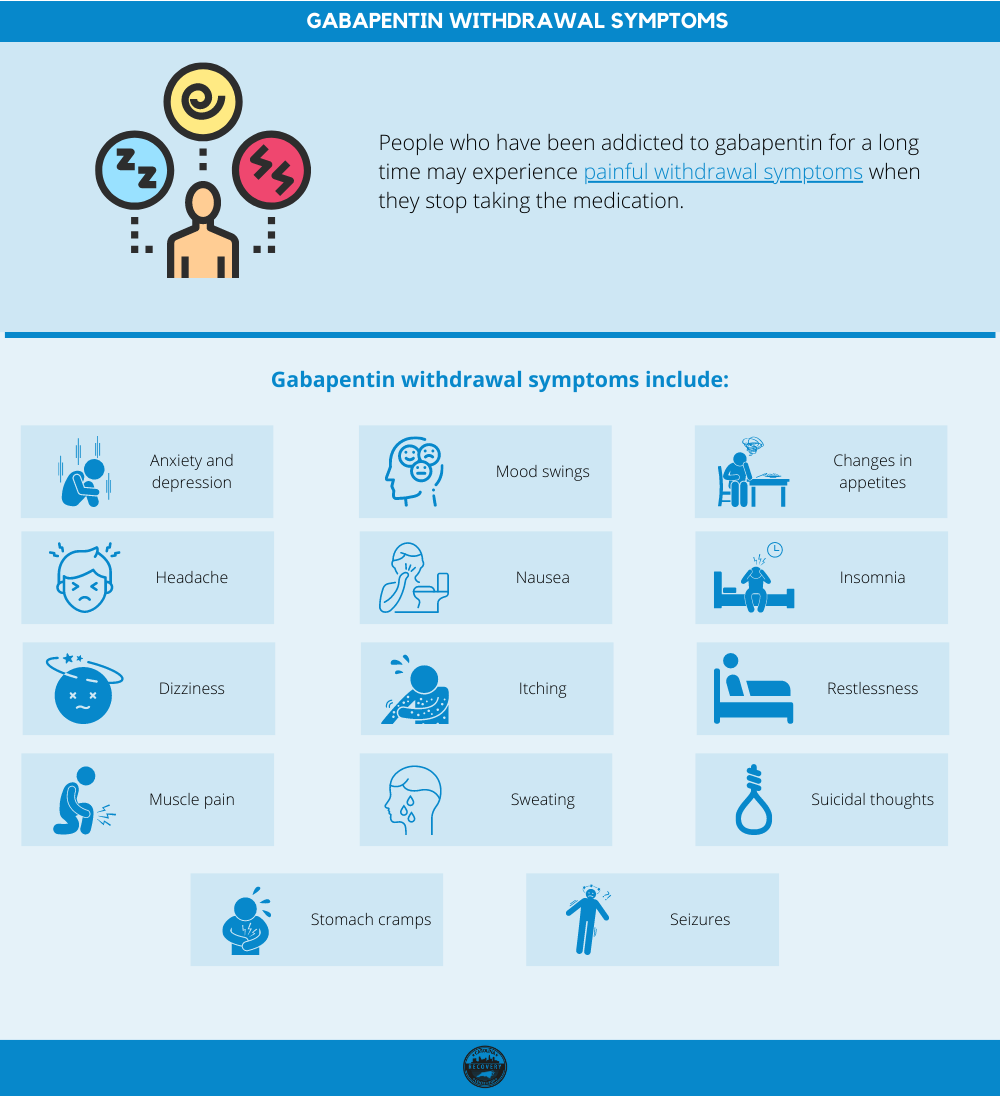
Request PDF | Neonatal Gabapentin Withdrawal Syndrome | Gabapentin, an anticonvulsant, neuroleptic, and pain medication, is widely used in both adults and children for management of epilepsy We report a retrospective case series of 19 infants exposed to both opioids and gabapentin prenatally. We describe a unique behavioral phenotype in 15 of these infants and report a treatment strategy. PURPOSE: Gabapentin, a gamma-aminobutyric acid (GABA) analog with antiepileptic and antinociceptive properties, is increasingly reported in the literature for the treatment of pain and agitation in medically complex patients in the neonatal intensive care unit (NICU), despite the paucity of safety and efficacy data in infants. The objectives of this study were to characterize gabapentin We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. Figure Neonatal abstinence scores were monitored using the Finnegan scoring system in the neonatal intensive care unit starting at 24 hours of life. Gabapentin treatment was initiated at approximately 124 hours of life (day of life 6) and the gabapentin dose was further escalated within the next 24 hours, as depicted by black arrows. Will it cause withdrawal symptoms in my baby after birth? Studies have not been done to see if gabapentin use alone can cause withdrawal in a newborn. One study found that when gabapentin is combined with opioids late in pregnancy, withdrawal can occur. %PDF-1.7 %âãÏÓ 112 0 obj > endobj xref 112 123 0000000016 00000 n 0000003281 00000 n 0000003486 00000 n 0000003527 00000 n 0000003562 00000 n 0000004020 00000 n 0000004126 00000 n 0000004241 00000 n 0000004349 00000 n 0000004463 00000 n 0000004571 00000 n 0000004686 00000 n 0000004791 00000 n 0000004906 00000 n 0000005013 00000 n 0000005128 00000 n 0000005235 00000 n 0000005350 00000 n Adverse events were reported in five of the 11 patients, and three were related to abrupt discontinuation of gabapentin due to NPO status, suggesting the existence of a gabapentin withdrawal syndrome in infants. We aimed to identify trends in gabapentin utilization among infants hospitalized in neonatal intensive care units (NICUs) across the United States (US) and to evaluate the associations between clinical diagnoses and gabapentin treatment. Introduction. Gabapentin is a gamma-aminobutyric acid analog that has been used in multiple disease states in children, including neuropathic pain, irritability, visceral hyperalgesia, neonatal abstinence syndrome (NAS), rescue sedation and feeding intolerance. 1–7 Despite the increased utilization of gabapentin in neonates, 1 there remains a gap in the pediatric literature describing the Gabapentin is an anticonvulsant, neuroleptic, and pain medication frequently used in adults and children with epilepsy, neuropathic pain, and neurological impairment. 2 Of note, there is no literature available on withdrawal symptoms in infants of gabapentin treated mothers, nor has the treatment of a newborn with signs of drug withdrawal from OBJECTIVE: Although gabapentin use is deemed safe during pregnancy, no clinical reports of gabapentin withdrawal syndrome in a neonate have been described. RESULTS: We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Gabapentin is an anticonvulsant, neuroleptic, and pain medication frequently used in adults and children with epilepsy, neuropathic pain, and neurological impairment. 2 Of note, there is no literature available on withdrawal symptoms in infants of gabapentin treated mothers, nor has the treatment of a newborn with signs of drug withdrawal from Results: We present a newborn who showed signs of withdrawal after prolonged in utero exposure to gabapentin. Clinical implications: Clinicians should be aware of possible withdrawal symptoms from drugs such as gabapentin, administered to mothers during pregnancy. The absolute risk for neonatal drug withdrawal was substantially higher among women who were co-exposed to opioids and psychotropic medications than among women exposed to opioids alone. Absolute risk of withdrawal in neonates ranged from 2.3% among women exposed to Z drugs to 11.4% among women exposed to gabapentin. An atypical withdrawal syndrome in neonates prenatally exposed to gabapentin and opioids. J Pediatr. 2017;181:286–288. doi: 10.1016/j.jpeds.2016.11.004. [ DOI ] [ PubMed ] [ Google Scholar ] A single case of withdrawal has been described in a neonate born to a mother on baclofen, gabapentin (1800 mg/day), and oxybutynin. The infant showed signs of drug withdrawal by 24 hours of life, including sneezing, irritability, jitteriness, and loose stools. Emerging concern exists regarding possible neonatal gabapentin withdrawal 31 after several studies contend a possibility of fetal retention following maternal exposure. 32-34 Huybrechts et al. 35 reported that in utero co-exposure to opioids and gabapentin was associated with both an increased risk of neonatal withdrawal and more severe Similar to most of the published studies with gabapentin in neonates and infants, no patients in our study were noted to have withdrawal symptoms after gabapentin discontinuation. Edwards et al 7 noted 3 children with withdrawal attributed to gabapentin including tachycardia, emesis, and irritability upon abrupt discontinuation. Maternal use of certain drugs during pregnancy can result in transient neonatal signs consistent with withdrawal or acute toxicity or cause sustained signs consistent with a lasting drug effect.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |