Gallery
Photos from events, contest for the best costume, videos from master classes.
.png) |  |
 |  |
 |  |
 |  |
 |  |
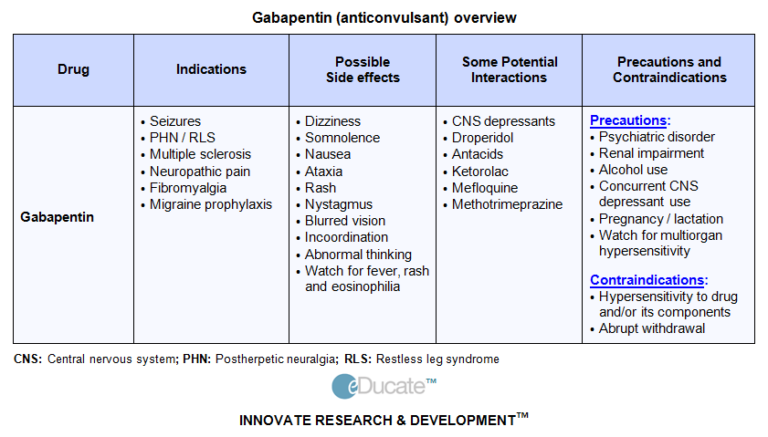 |  |
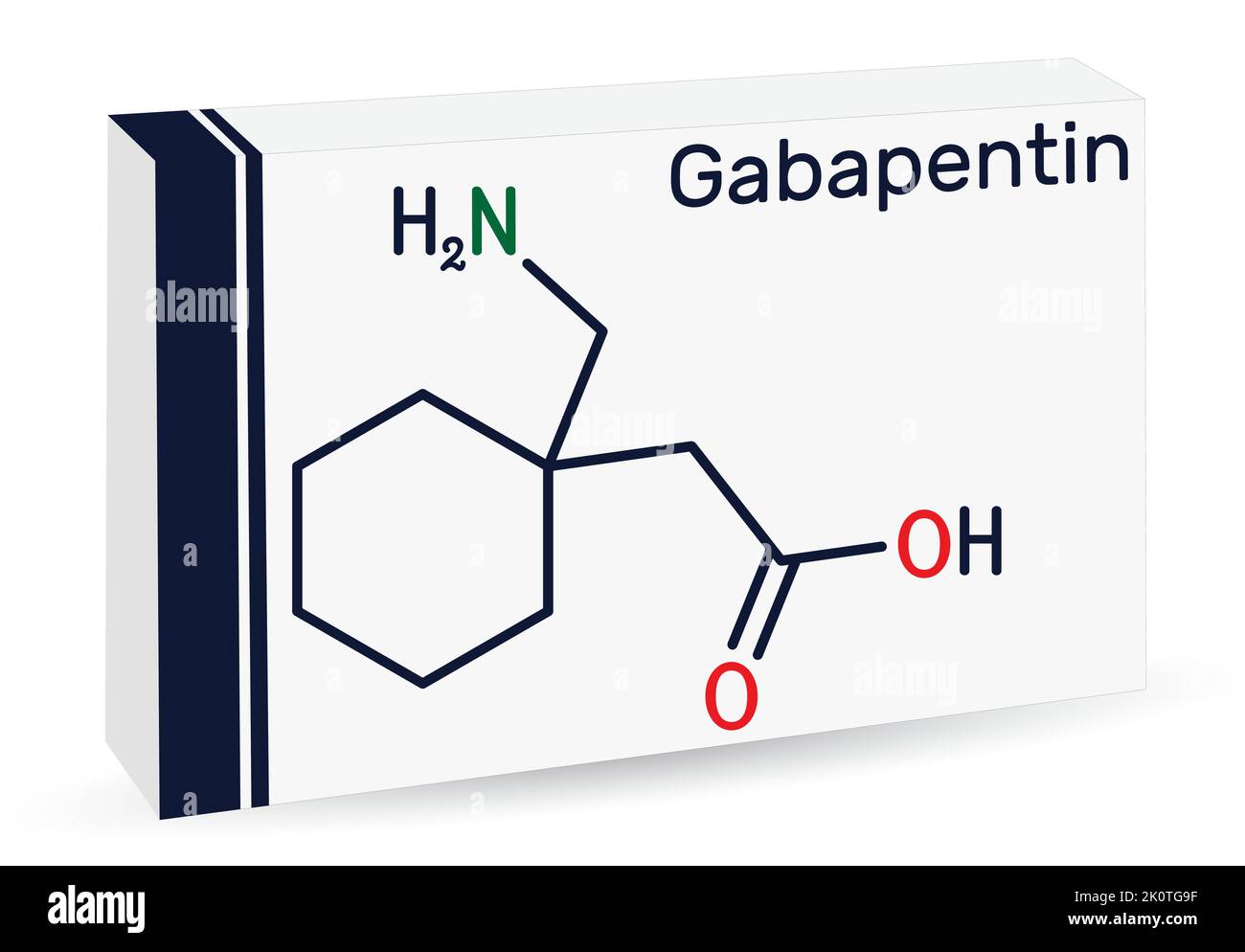
Gabapentin was first approved for use in the United Kingdom in 1993. [16] . It has been available as a generic medication in the United States since 2004. [17] . It is the first of several other drugs that are similar in structure and mechanism, called gabapentinoids. Gabapentin can be efficacious when co-prescribed in the management of pain or pain-related conditions, AUD, and some forms of anxiety disorders, but gabapentin can be dangerous when used with CNS depressants or in high doses alone. 30,31 Prescribers may be able to minimize the increasing misuse of gabapentin and improve patient safety by Case Histories. A.J. Thorpe, C.P. Taylor, in Comprehensive Medicinal Chemistry II, 2007 8.18.3 Clinical Development of Gabapentin 8.18.3.1 Early Clinical Studies. Gabapentin was studied in animal toxicology at Goedecke from about 1980 to 1982, and was first studied for tolerance, safety, and pharmacokinetics in healthy human subjects in a study contracted from Goedecke in 1982. 29 Clinical Age: 3 to 11 years: Initial dose: 10 to 15 mg/kg/day orally in 3 divided doses Maintenance dose: Age: 3 to 4 years: 40 mg/kg/day orally and in 3 divided doses (3 times a day) Age: 5 to 11 years: 25 to 35 mg/kg/day in 3 divided doses (3 times a day) Maximum dose: Doses up to 50 mg/kg/day have been well tolerated in a long term clinical study Gabapentin is a prescription medication that was approved by the U.S. Food and Drug Administration in 1993 as a treatment for epilepsy. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures - today it is also widely used to treat neuropathic pain. Gabapentin is approved for treatment of focal seizures Among 49 case reports submitted to the FDA over the five-year period from 2012 to 2017, twelve people died Gabapentin, a GABA receptor agonist, was first studied as an antiepileptic drug in humans in 1987 (07). It was launched in the United Kingdom in 1993 and approved in the United States as add-on therapy for intractable partial seizures in adults. It is also approved for the treatment of postherpetic neuralgia. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8,10 Gabapentin has Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology. Introduction. The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The Gabapentin was discovered by Gerhard Satzinger. Based at Parke-Davis’s German labs, he set up the GABA project with aim to create drug molecules targeting the neurotransmitter of the These marketing tactics came at a settlement price of US $430 million in criminal and civil liability charges in 2004, 40,43 but led to a tremendous growth in gabapentin prescriptions for off-label use from the early 1990s to early 2000s, 40 a trend that has now shaped modern practice. 44 After the settlement, use of gabapentin for off-label Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Gabapentin was discovered 40 years ago by a Japanese scientist named Gerhard Satzinger. Initially, he was looking for a muscle relaxant or antispasmodic. Later in 2000, Parke-Davis bought it and contacted several tests on it to find out its effectiveness in treating people with epilepsy. Gabapentinoids, also known as α2δ ligands, are a class of drugs that are chemically derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) (i.e., GABA analogues) which bind selectively to the α 2 δ protein that was first described as an auxiliary subunit of voltage-gated calcium channels (VGCCs). [1][2][3][4][5] Gabapentin is a nonprotein amino acid and a synthetic neurotransmitter that is related to γ-aminobutyric acid 1 (GABA). It was first described in 1976 West German patent DE2460891 on cyclic amino acids to Gerhard Satzinger and co-inventors at Goedecke AG (Freiburg). Gabapentin and pregabalin for pain—is increased prescribing a cause for concern?. N Engl J Med. 2017;377(5) transmitted or reproduced in any medium, whether now known or later invented Max dosage 3600mg if patient already on gabapentin; Taper dose > 7 days to discontinue; Pediatric Dosing Partial seizures. Adjunct for partial seizures with out secondary generalization in patients> 12yo with epilepsy; also adjunctive therapy for partial seizures in patients 3-12 years <3 years: Safety and efficacy not established The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
.png) |  |
 |  |
 |  |
 |  |
 |  |
 |  |