Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
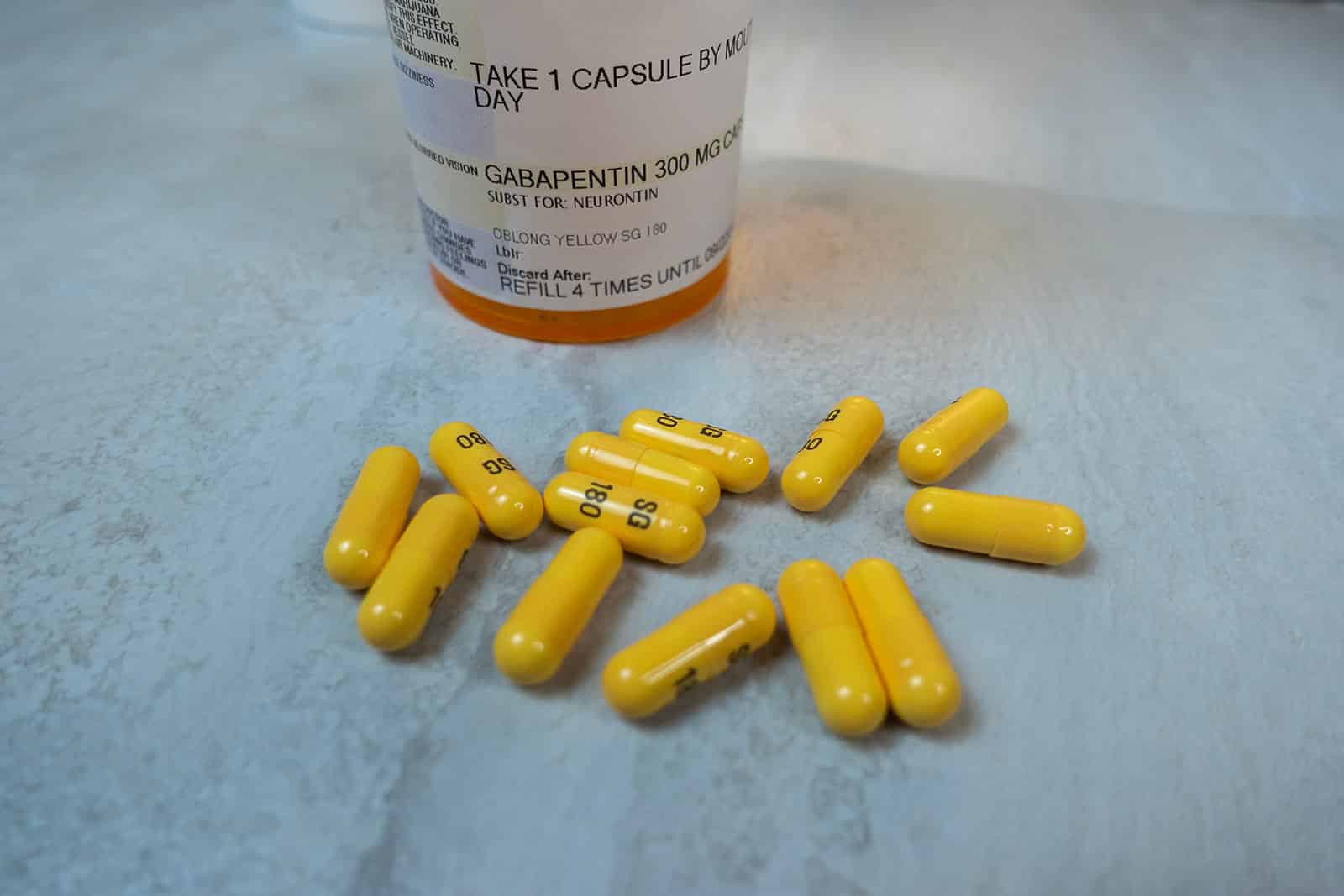 |  |
 |  |
 |  |
 |  |
 |  |
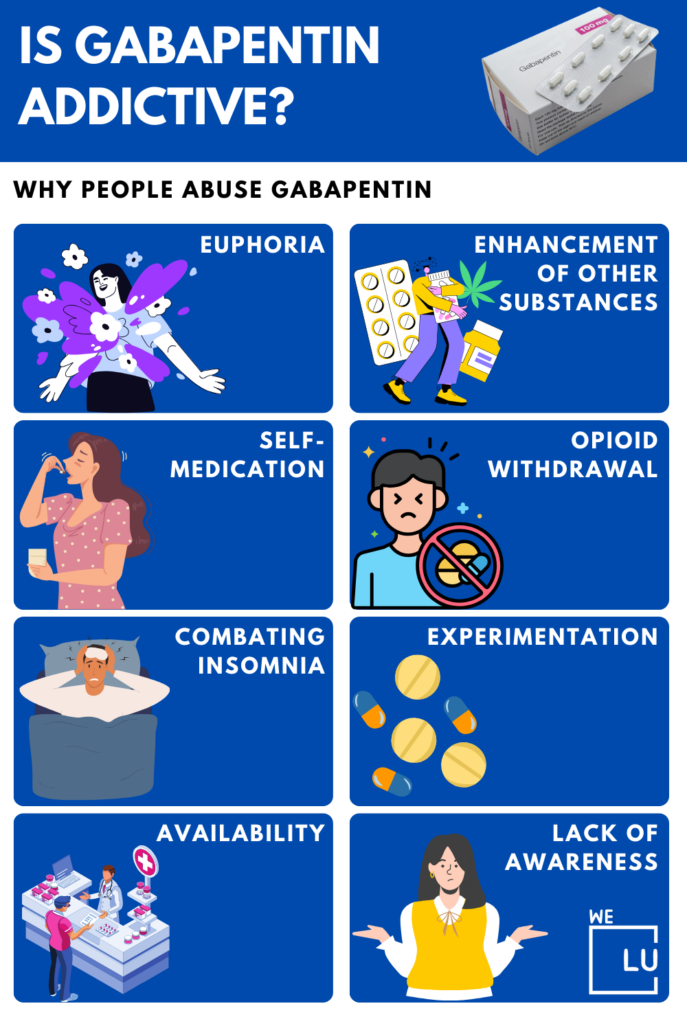
, any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Gabapentin is not a controlled substance under the federal Controlled Substances Act. [125] Effective 1 July 2017, Kentucky classified gabapentin as a schedule V controlled substance statewide. [126] Gabapentin is scheduled V drug in other states such as West Virginia, [127] Tennessee, [128] Alabama, [129] Utah, [130] and Virginia. [131] A controlled substance prescription must be issued by a DEA registered practitioner. 21 C.F.R. § 1306.03(a) A controlled substance prescription must be issued for a legitimate medical purpose. 21 C.F.R. 1306.04(a ) Controlled Substance Prescription Requirements U.S. Drug Enforcement Administration Diversion Control Division Each prescription for a controlled substance or dangerous drug must be maintained in a separate file pursuant to the requirements set forth in NAC 453.480. (d) Keep all controlled substances and dangerous drugs in a locked storage area. Access to the storage area must be restricted to the persons described in NRS 453.375. The FDA approved gabapentin in 1993 as a non-controlled substance and it has remained a non-controlled substance at the federal level. The drug was created as an anticonvulsant and used to treat seizure disorders. After 5 refills or after 6 months, whichever occurs first, a new prescription is required. This rule applies to all controlled substances in schedule III and IV. 2. Tramadol is associated with a wide variety of side effects. Tramadol has a long list of serious and potentially deadly side effects. Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. R 338.3125 - Gabapentin has been added to the schedule 5 drug list as a controlled substance. As a result of this change, any prescribers prescribing gabapentin must be registered with the Michigan Automated Prescription System (MAPS). Gabapentin is not a controlled substance on a federal level but is controlled in some states, which limits the number of prescription refills and how it is reported. Gabapentin can be dangerous when used in combination with other substances, particularly opioids. 36. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance. 37. I suspect my healthcare practitioner is engaged in TennCare fraud, waste, or abuse. What do I do? For individuals applying for their initial controlled substance license, as well as those renewing their controlled substance license, rule changes made to R 338.3135 now require continued training in opioids and controlled substance awareness before applying for the initial license and each subsequent renewal of a controlled substance license. • Prescriptions for gabapentin may not be pre-signed or post-dated. How does moving gabapentin to Schedule 5 affect dispensing practitioners? • Only authorized practitioners may directly dispense controlled substances to patients. In Kentucky, no mid-level practitioners are authorized to directly dispense controlled substances. In the US, gabapentin was approved by the Food and Drug Administration as a non-controlled substance. To date, and in spite of empirical evidence suggestive of diversion and abuse with opioids, gabapentin remains a non-controlled substance at the federal level. “Gabapentin has been dangerously unscheduled as a controlled substance for too long despite increasing evidence of abuse and misuse and despite its strong similarity to pregabalin, which has been a schedule V drug for more than 15 years,” said Michael Abrams, senior health researcher with Public Citizen’s Health Research Group and lead Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. o Any prescription written for a controlled substance in high doses or high quantities o Any prescription considered an outlier to what is normally prescribed (use clinical judgment) • Additional recommendations: o It is recommended that pharmacists document a note 2 ARIZONA GUIDELINES FOR DISPENSING CONTROLLED SUBSTANCES A pharmacist has reason to believe the patient has received prescriptions for controlled substances from more than one prescriber in the preceding three months, unless the prescriptions are from prescribers who practice at the same physical location (i.e. same group practice) 6. Patient is exhibiting signs of potential abuse or diversion. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |