Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 | |
 | 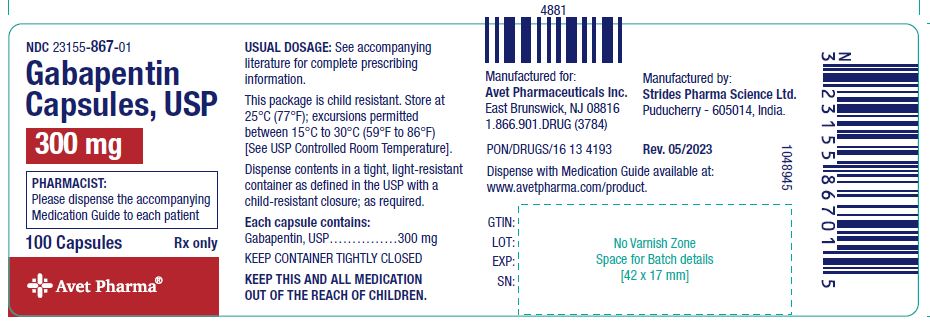 |
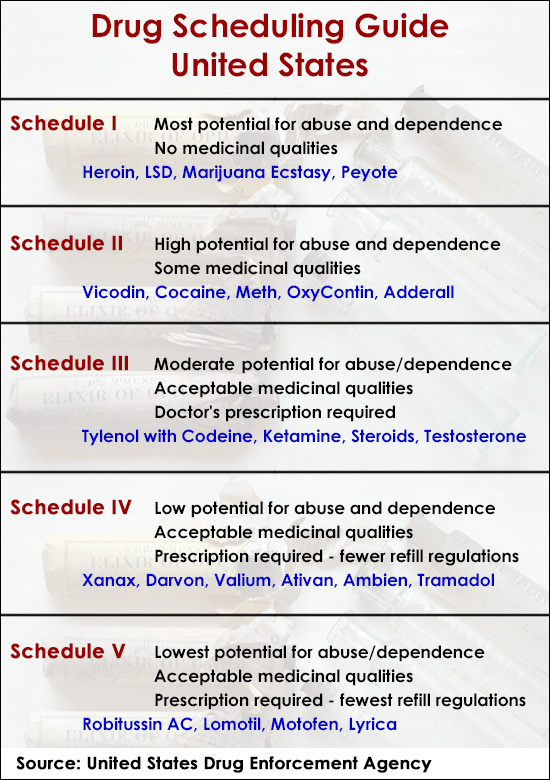
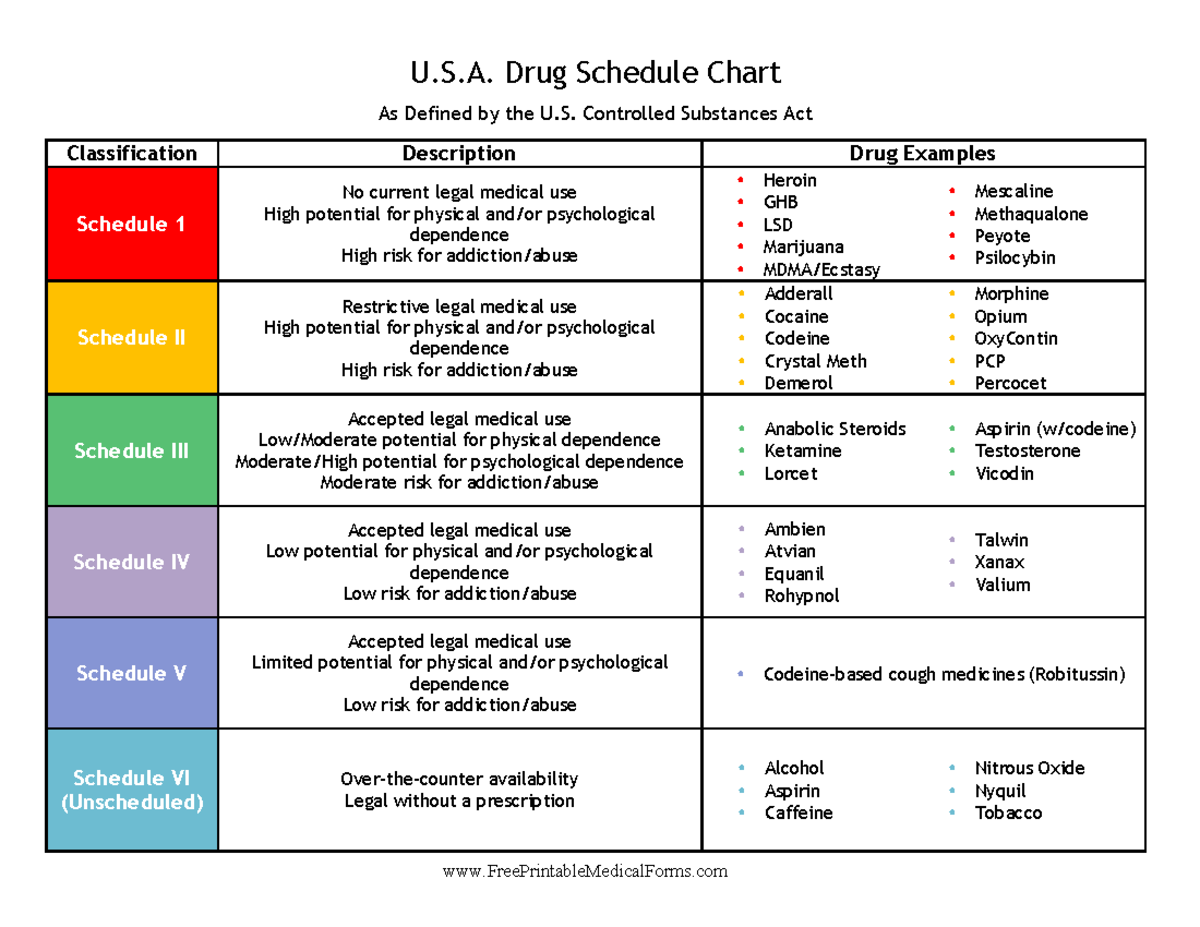
In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky As of November 2020, seven states — Alabama, Kentucky, Michigan, North Dakota, Tennessee, Virginia, and West Virginia — had classified gabapentin as a schedule V drug, while another 12 36. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance. 37. I suspect my healthcare practitioner is engaged in TennCare fraud, waste, or abuse. What do I do? Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. policies on filling of controlled substances. Prescriptions dated on or after July 1, 2018, should be treated as controlled substance prescriptions and contain all controlled substance requirements listed above. Remember to review all controlled substance record keeping, inventory, prescribing and dispensing requirements as outlined by the DEA Effective July 1, 2017, all gabapentin products will be Schedule 5 controlled substances in Kentucky. All applicable provisions of KRS Chapter 218A, 902 KAR Chapter 55 and other licensure board regulations will apply to gabapentin. Please review all controlled substance security, storage, record Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and States acknowledge that gabapentin has abuse potential Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because it may cause a level of sedation in patients that puts them at increased risk of overdose when taken with opioids. The NC Department of Health and Human Services (NCDHHS) has Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. As of writing, there are at least five states that have reclassified gabapentin as a controlled substance. There are also some states that are closely monitoring the use of gabapentin and have put the drug through PDMPs or prescription drug monitoring programs. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Though the U.S. Drug Enforcement Agency has not reclassified gabapentin, 7 state boards of pharmacy are now acknowledging gabapentin’s abuse potential and have reclassified gabapentin as a Schedule V controlled substance. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled substance because of its abuse potential as a non-opioid pain reliever . iii At least 26 States and the District of Columbia have enacted legislation or introduced a bill to schedule gabapentin as a controlled substance or Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 | |
 |  |