Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
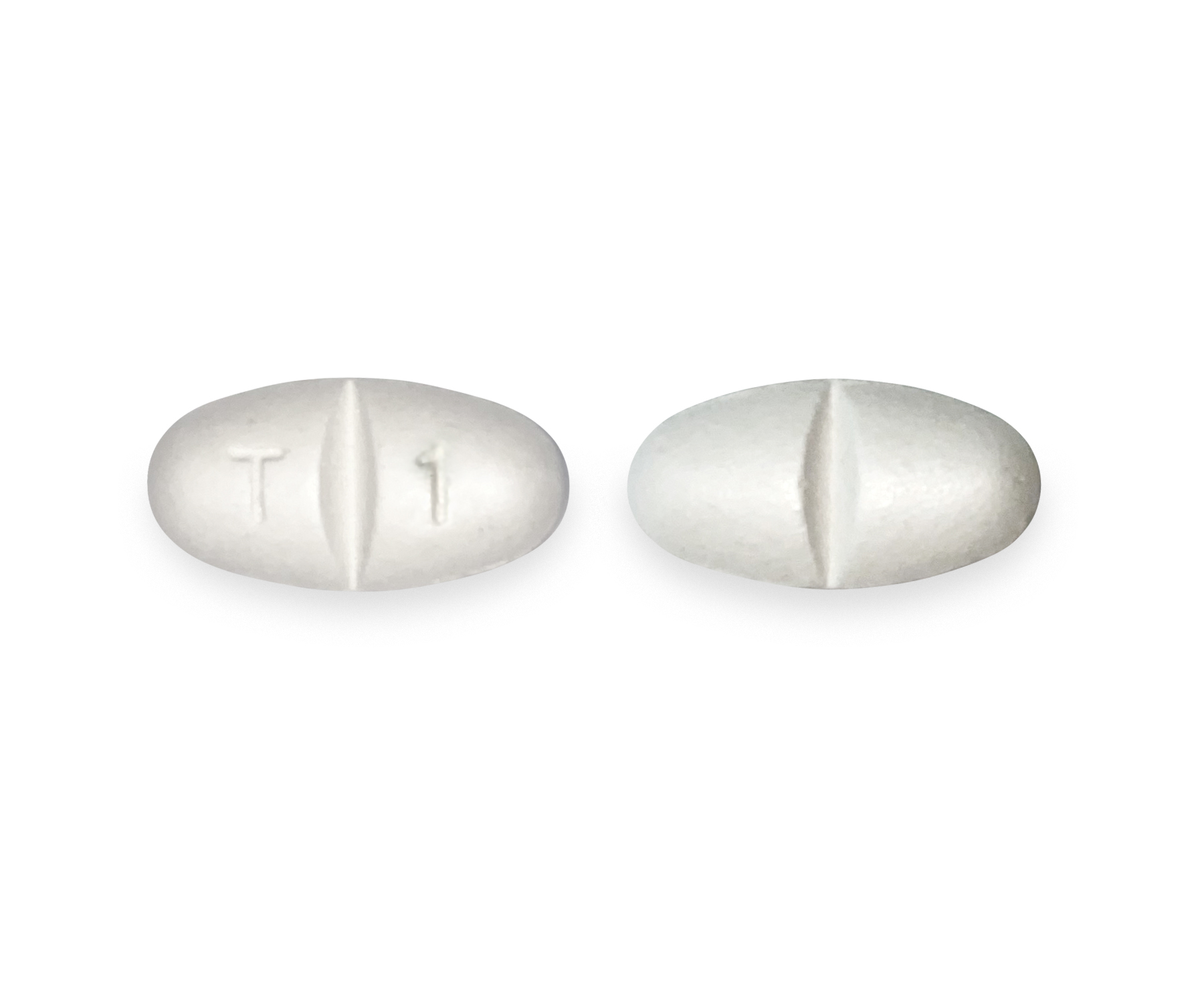
Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Neurontin (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Geriatrics (>65 years of age) Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic acid with an empirical formula of C9 H17NO2 and a molecular weight of 171.24. The molecular structure of gabapentin is: Gabapentin What are the ingredients in gabapentin? Active ingredient: Gabapentin USP . Inactive ingredients in the capsules: anhydrous lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin, sodium lauryl sulfate, and titanium dioxide. Do not stop taking gabapentin without first talking to your healthcare provider. Stopping gabapentin suddenly can cause serious problems. Gabapentin can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch, gelatin, magnesium stearate, manni What are the ingredients in NEURONTIN? Active ingredient: gabapentin . Inactive ingredients in the capsules: lactose, cornstarch, talc, gelatin, titanium dioxide and FD&C Blue No. 2. The 300-mg capsule shell also contains: yellow iron oxide. The 400-mg capsule shell also contains: red iron oxide, and yellow iron oxide. Neurontin is a prescription medicine used to treat: Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. Partial seizures when taken together with other medicines in adults and children 3 years of age and older with seizures. of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. The 100 mg capsule shell contains gelatin and titanium dioxide. The 300 mg capsule shell contains gelatin, titanium dioxide, and yellow iron oxide. Nonmedicinal ingredients: cornstarch, lactose, and talc; capsule shell: FD&C Blue No. 2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide. The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin,which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. Gabapentin capsules are a prescription medicine used to treat: • • Who should not take gabapentin capsules? Do not take gabapentin capsules if you are allergic to gabapentin or any of the other ingredients in gabapentin capsules. See the end of this Medication Guide for a complete list of ingredients in gabapentin capsules. NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. Store NEURONTIN Capsules between 59°F to 86°F (15°C to 30°C). Store NEURONTIN Tablets between 59°F to 86°F (15°C to 30°C). Store NEURONTIN Oral Solution in the refrigerator between 36°F to 46°F (2°C to 8°C). Keep NEURONTIN and all medicines out of the reach of children. General information about the safe and effective use of NEURONTIN The inactive ingredients for the oral solution are glycerin, xylitol, purified water and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic acid Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only). NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Key Takeaways: Ingredients of Gabapentin Active Ingredient: Gabapentin is the primary component for nerve pain relief. Inactive Ingredients Matter: They influence absorption and potential side effects. Diverse Dosage Forms: Options include tablets, capsules, and oral solutions. Kidney Function Impact: Renal health affects gabapentin metabolism and dosage. Patient Education is Key:Patient
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |