Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
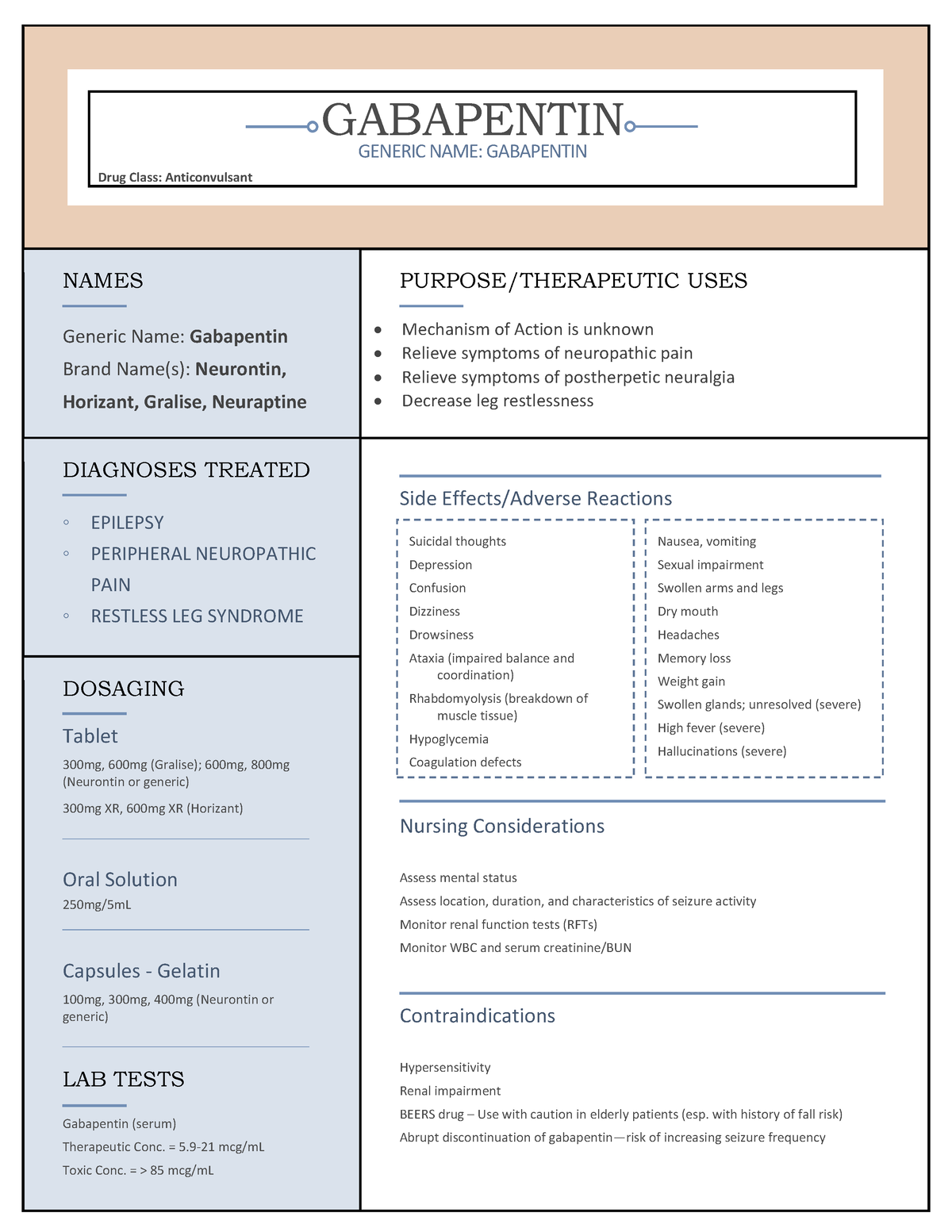
Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Find information on Gabapentin (Gralise, Horizant) in Davis’s Drug Guide including dosage, side effects, interactions, nursing implications, mechanism of action, half life, administration, and more. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin Conventional (immediate-release) Capsules and Tablets. 25°C (may be exposed to 15–30°C). Gabapentin Conventional (immediate-release) Oral Solution. 2–8°C. Gabapentin Gastroretentive Tablets (Gralise) 25°C (may be exposed to 15–30°C). Gabapentin Enacarbil Extended-release Tablets (Horizant) 25°C (may be exposed to 15–30 Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin belongs to a class of drugs called anticonvulsants. These drugs are used to treat a variety of conditions caused by abnormal electrical activity in the brain, including seizures, nerve pain, and restless leg syndrome. Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin is an anticonvulsant prescription drug that goes by several brand names including, Neurontin, Gralise, Gabarone, and Fanatrex. It was approved by the FDA in December 1993 for the Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . What Is Gabapentin and How Does It Work? Gabapentin is a prescription drug most commonly prescribed to relieve nerve pain following shingles in adults, treating the pain of post herpetic neuralgia. Gabapentin belongs to a class of drugs known as anti- seizure drugs. Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Neurontin is used in adults to treat neuropathic pain (nerve pain) caused by herpes virus or shingles (herpes zoster). Neurontin is also used to treat seizures in adults and children who are at least 3 years old. Use only the brand and form of gabapentin your doctor has prescribed. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section GABAPENTIN (Trade Name: Neurontin ®) Introduction: Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. (a) Any compound, mixture, or preparation containing any of the following limited quantities of narcotic drugs, which shall include one or more nonnarcotic active medicinal ingredients in sufficient proportion to confer upon the compound, mixture, or preparation valuable medicinal qualities other than those possessed by the narcotic drug alone: In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada , or Australia , and the other gabapentinoids, including phenibut, are not controlled substances
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |