Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 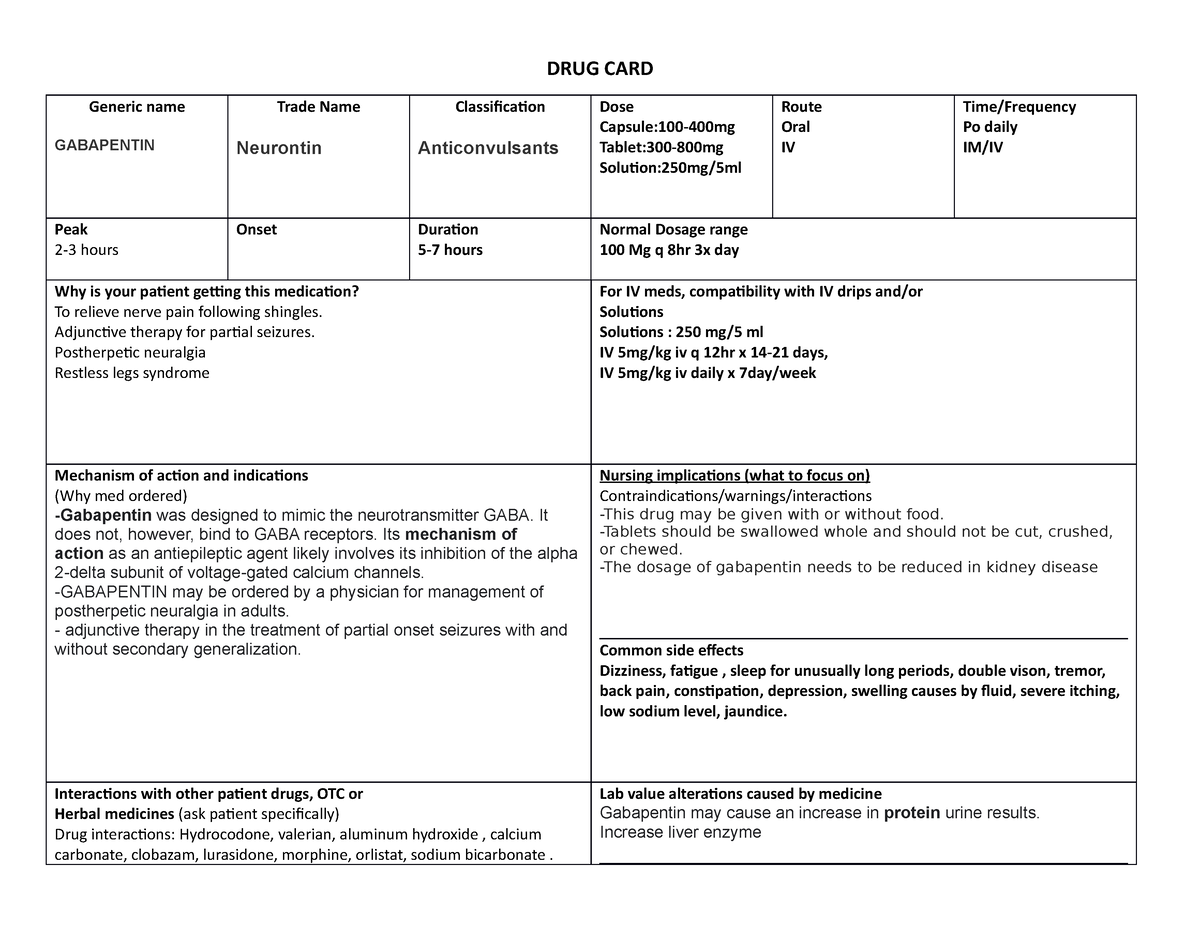 |
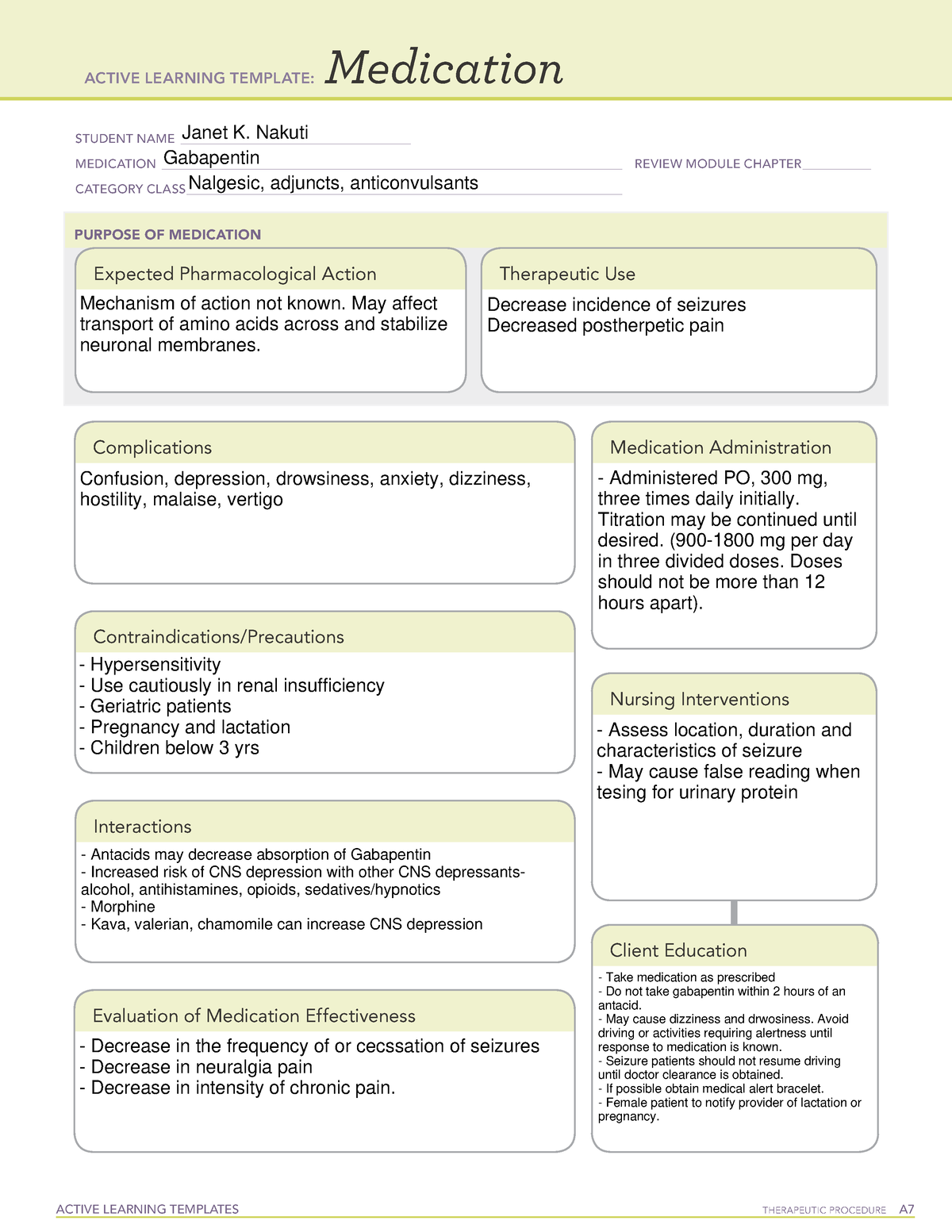
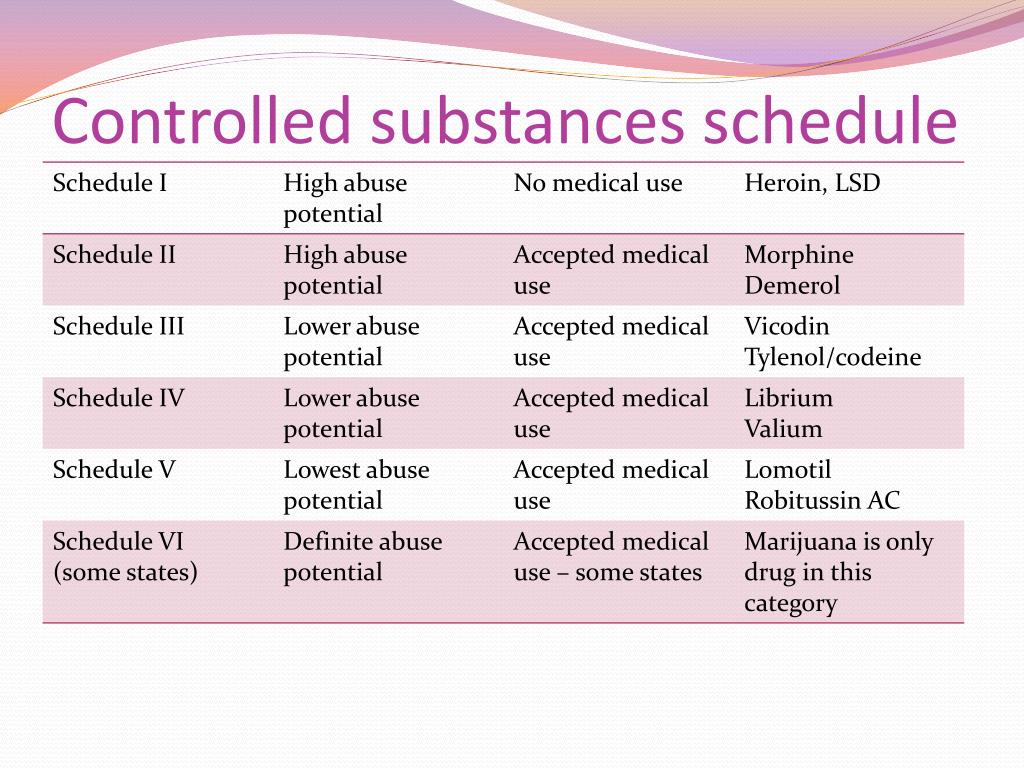
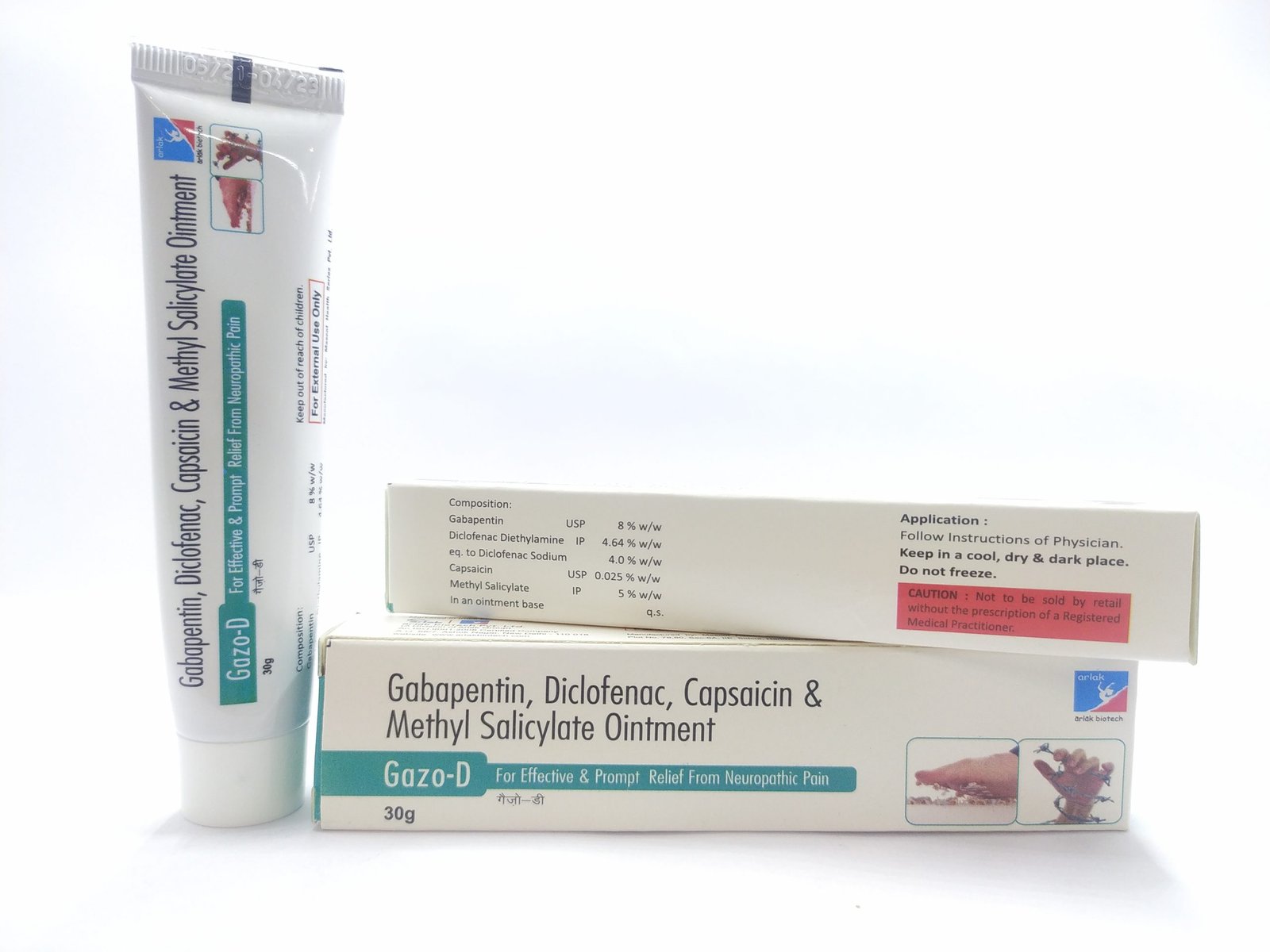
Pregabalin and gabapentin are due to become controlled substances following a recent increase in the number of deaths attributed to the drugs. Last year, the Advisory Council on the Misuse of Drugs (ACMD) wrote to the government recommending the drugs to be reclassified as class C drugs because of the risks of “illegal diversion and medicinal Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentinoids, also known as α2δ ligands, are a class of drugs that are chemically derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) (i.e., GABA analogues) which bind selectively to the α 2 δ protein that was first described as an auxiliary subunit of voltage-gated calcium channels (VGCCs). [1][2][3][4][5] Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin is in a class of medications called anticonvulsants. What are the brand names of gabapentin? Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. Class C includes: certain drugs related to the amfetamines such as benzfetamine and chlorphentermine, Z-drugs (e.g. zopiclone, zolpidem), opioids, gabapentin, and (8) Any synthetic opioid controlled in Schedule I of 21 C.F.R. 1308.11 or Schedule II of 21 C.F.R. 1308.12, unless specifically excepted or unless listed in another class in this section. CLASS B to reflect growing concern about abuse, both pregabalin and gabapentin are now classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 (as amended) as Schedule 3, but are exempt from the safe custody requirements (see new legal requirements and resources for Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Today’s move comes after experts At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Find information on Gabapentin (Gralise, Horizant) in Davis’s Drug Guide including dosage, side effects, interactions, nursing implications, mechanism of action, half life, administration, and more. Davis Drug Guide PDF. The Misuse of Drugs Act 1971 (Amendment) Order 2018 classifies pregabalin and gabapentin as Class C drugs under paragraph 1 (b) of Part 3 of Schedule 2 to the Misuse of Drugs Act 1971 (“the Taking gabapentin with other drugs that make you drowsy or slow your breathing can cause dangerous side effects or death. Ask your doctor before taking opioid medication, a sleeping pill, a muscle relaxer, or medicine for anxiety or seizures. Tell your doctor about all your current medicines. Many drugs can affect gabapentin, especially: naproxen; From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Pregabalin and gabapentin are to be reclassified as Class C controlled substances from April 2019, the government has announced. The switch will come more than two years after the Advisory Council on the Misuse of Drugs recommended that the two medicines become controlled drugs and be placed under Schedule 3 of the Misuse of Drugs Regulations Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Food does not significantly affect the T max of gabapentin and increases the C max and area-under-curve levels of gabapentin by approximately 10%. [94] Gabapentin can cross the blood–brain barrier and enter the central nervous system. [85] Gabapentin concentration in cerebrospinal fluid is approximately 9–14% of its blood plasma From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin For generic drugs, if the labeling of a reference listed drug is updated as a result of the final rule, the abbreviated new drug application (ANDA) labeling must also be revised. Labeling for over-the-counter (OTC) medicines will not change, as OTC drug products are not affected by the new FDA pregnancy labeling.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |