Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
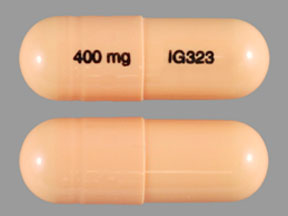 |  |
Florida’s Prescription Drug Monitoring Program, known as E-FORCSE® (Electronic-Florida Online Reporting of Controlled Substances Evaluation) is a database that collects and stores schedule II-V controlled substance dispensing information. Authorized users are able to access patient histories to provide medical treatment to a current patient. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Florida has strict laws governing the handling of controlled substances in pharmacies to prevent misuse, abuse, and diversion. These regulations dictate how these drugs are classified, prescribed, dispensed, and stored, ensuring patient safety and legal compliance for healthcare providers. Section 456.0301, Florida Statutes, requires all physicians who are registered with the U.S. Drug Enforcement Agency and authorized to prescribe controlled substances to complete a board approved 2-hour course on prescribing controlled substances. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin is not a narcotic or federally controlled substance, but it is a Schedule V drug in certain states. Learn why gabapentin is controlled, which states regulate it, and how it can interact with opioids and other drugs. If gabapentin is, or becomes, a controlled substance in your state, it does not necessarily mean it will be more difficult to obtain. Rather, it is a safety measure to assure we are using medications appropriately. Chronic pain and opioid use and abuse is a significant problem in the United States and in Florida.[1] Over one-quarter of United States citizens suffer from chronic pain.[2] It is among the most common complaints seen in an outpatient clinic and the emergency department. The failure to manage chronic pain, as well as the possible complication of opioid dependence related to treatment, can — A substance in Schedule I has a high potential for abuse and has no currently accepted medical use in treatment in the United States and in its use under medical supervision does not meet accepted safety standards. The following substances are controlled in Schedule I: 1. Acetyl-alpha-methylfentanyl. 2. Acetylmethadol. 3. Allylprodine. 4. gabapentin is prescribed in lieu of opioids due to its trivial potential for addiction and abuse. In 2017, it was the 10. th. most commonly prescribed medication with 68 million prescriptions dispensed. 4. The misuse of gabapentin can be easily overlooked since it’s not a controlled substance and is easily dispensed in large quantities at a time. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food Here are some of the drugs included in the Controlled Substances bill. Health providers have to check the state database before writing a prescription for these drugs. Only Schedule II drugs Gabapentin is a prescription medication approved by the United States Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. — A substance in Schedule I has a high potential for abuse and has no currently accepted medical use in treatment in the United States and in its use under medical supervision does not meet accepted safety standards. The following substances are controlled in Schedule I: 1. Acetyl-alpha-methylfentanyl. 2. Acetylmethadol. 3. Allylprodine. 4. 893.135 involving any controlled substance described in subparagraph 3. or subparagraph 4., the controlled substance is a Schedule III controlled substance pursuant to this paragraph but the weight of the controlled substance per milliliters or per dosage unit is not relevant to the charging of a violation of s. 893.135. The weight of the Gabapentin is not a certified controlled substance, but it is still addictive. Treatment may help those living with a gabapentin use disorder. Gabapentin is commonly prescribed to treat seizures in adults and children. It can also treat nerve pain in adults, but it is associated with a risk for abuse. As prescribers across the country unite [] Gabapentin is frequently combined with other substances for the purpose of potentiating the effects of the drugs or achieving a “high.” Studies have identified various substances that are commonly abused in combination with gabapentin, including alcohol, opioids, benzodiazepines, antidepressants, and other CNS depressants 13,14,15.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |