Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
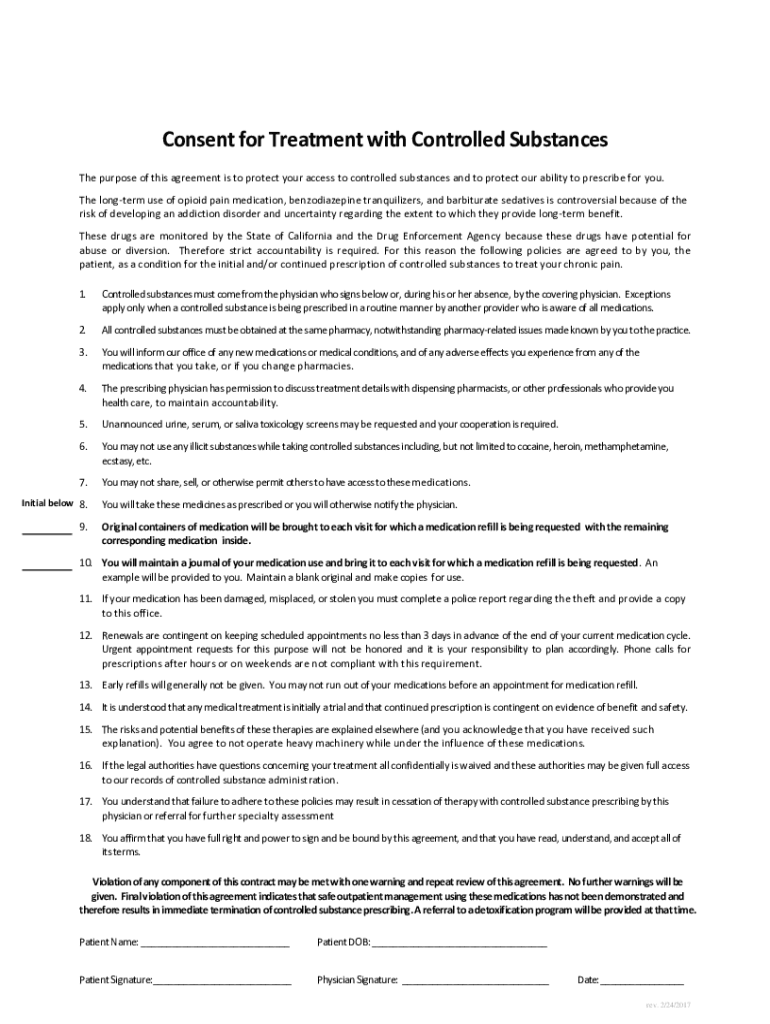
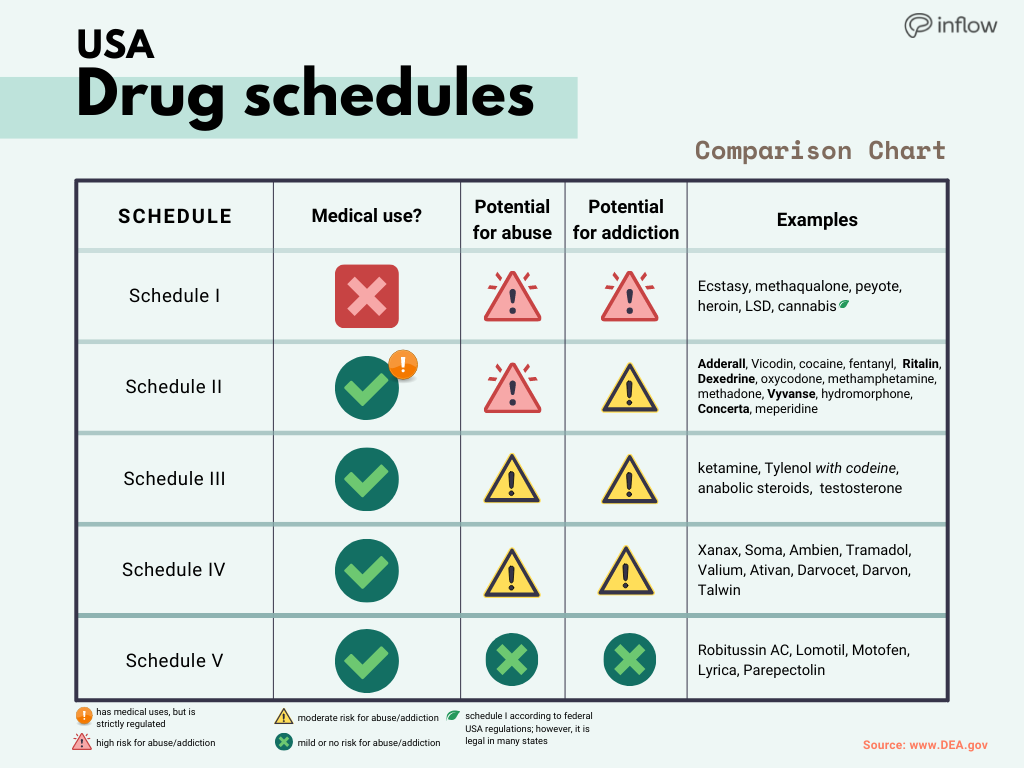
The number of individuals who have Gabapentin present in an accidental overdose is significant enough . to add Gabapentin dispensation into the CPRMS. Note: The Department is not changing the controlled substance scheduling of Gabapentin at this time. As such, the CPMRS look-up requirements do not apply to Gabapentin prescribing. Naloxone schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. In Indiana, gabapentin’s classification has been scrutinized due to its misuse potential. Although not classified under the federal Controlled Substances Act, the Indiana Board of Pharmacy designated gabapentin as a “drug of concern” in 2019. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Indiana’s classification of controlled substances is governed by the Indiana Code Title 35, Article 48, aligning with the federal Controlled Substances Act. The state categorizes drugs into five schedules based on potential for abuse, accepted medical use, and safety under medical supervision. The Senate Corrections and Criminal Law Committee heard HB 1182 on regulation of controlled substances authored by Rep. McNamara and sponsored by Sen. Glick. The bill relocates requirements that the board of pharmacy must comply with when adopting interim rules declaring a substance a synthetic drug from the professional licensing law to the statutes governing administrative rulemaking. The short answer is, based on the language of Indiana Code § 35-48-7-11.3 and the guidance of the Professional Licensing Agency, it appears that any veterinarian that distributes, dispenses, or prescribes a controlled substance is required to register for INSPECT, and the IPLA requires that each practitioner INSPECT registrant have their own individual DEA License to register. The INSPECT program monitors all prescriptions for ephedrine, pseudoephedrine, and controlled substances. "Controlled Substance" has the meaning set forth in IC 35-48-1-9. The term includes gabapentin. Click here for a list of the drugs as described in the Federal Controlled Substances Act. Am I permitted to share INSPECT information? Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to January 17, 2025 Filed Under: Criminal. The House Courts and Criminal Code Committee heard HB 1056 authored by Rep. McNamara and by Rep. Steuerwald on controlled substances. The bill adds 2-Methyl AP-237 to the list of schedule I controlled substances. Statutory Changes: Updated on March 2025. S.L. 2023-65 amended G.S. 90-113.73(b) Adds Gabapentin to the list of substances to be reported into the CSRS, by dispensers, effective March 1, 2024; this law requires veterinarians to report prescriptions of Gabapentin effective March 1, 2025. FAQ’s for Dispensers- Effective March 1, 2024 However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Other states are recognizing the growing abuse problem with gabapentin and have, at the very least, mandated that it be included in their prescription drug monitoring programs. Controlled Substances July 1, 2016: Pursuant to Indiana Code 25-26-13-4, ephedrine and pseudoephedrine, when dispensed via prescription, are each considered a "controlled substance" for the purposes of the INSPECT program. All prescription dispensations of these drugs must be reported to INSPECT. 2020 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5. 2024 Indiana Code Title 25. Professions and Occupations Article 26. Pharmacists, Pharmacies, Drug Stores Chapter 24. Central Repository for Controlled Substances Data 25-26-24-2.5.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |