Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |
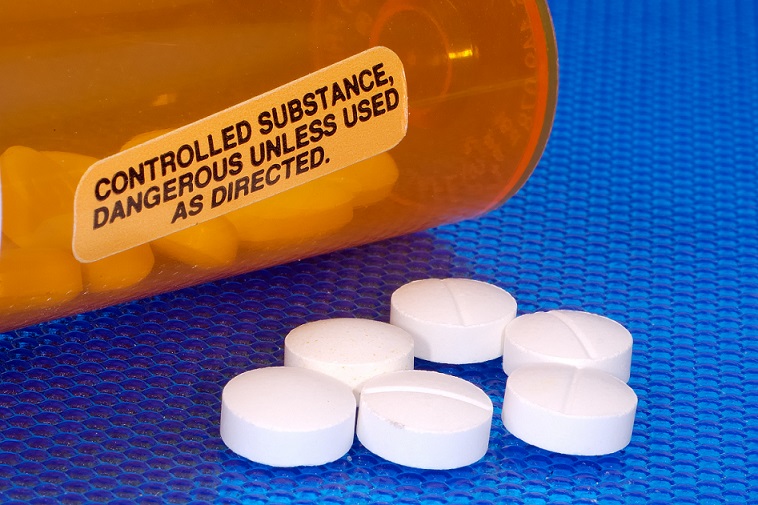
Ohio classifies controlled substances into five schedules under the Ohio Revised Code 3719.41, mirroring the federal Controlled Substances Act. These schedules are determined based on a substance’s potential for abuse, accepted medical use, and likelihood of dependence. However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Other states are recognizing the growing abuse problem with gabapentin and have, at the very least, mandated that it be included in their prescription drug monitoring programs. In 2020, a federal court case in Ohio examined gabapentin’s role in the opioid crisis. While the court did not reclassify gabapentin, it emphasized the need for ongoing monitoring and regulation at both state and federal levels. Effective March 1, 2025 On March 1, 2025, the following rules will go into effect: • Rule 4729:5-3-22 of the OAC requires any pharmacy licensed as a TDDD to implement a continuous quality improvement program for pharmacy services. For more information on the requirements of this rule, visit www.pharmacy.ohio.gov/ PharmacyCQI. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Any licensee engaged in the transfer, including intracompany transfers, or sale of controlled substances or gabapentin must report those transactions to OARRS. Any transfer of a controlled substance or gabapentin between two different TDDD license numbers (or DEA registrations) must be reported as a wholesale transaction, even if just one tablet. schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin Dosage and Administration. Gabapentin is available in multiple forms, including capsules, tablets, and a liquid solution. Patients typically begin with a low dose, which is gradually increased under medical supervision based on symptoms and how well the medication is tolerated. Gabapentin has not been reclassified as a controlled substance, but it is being added to the Board’s list of drugs reportable to OARRS following increased reports of misuse, abuse, and concomitant abuse of gabapentin nationwide. 1. No new requirement to review an OARRS report prior to dispensing gabapentin Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. registration numbers for gabapentin prescriptions issued by veterinarians. As a reminder, gabapentin is not a controlled substance in Ohio and so a veterinarian is not required to have a DEA registration number to prescribe the medication. Additionally, veterinarians do not have NPI numbers. controlled substances schedules. (Title 21, CFR 1308.31.) Accordingly, these drugs are legally classified as dangerous drugs in Ohio. Rx-Prescription Drugs. 77 South High Street, 17th Floor, Columbus, Ohio 43215 . T: (614) 466.4143 | F: (614) 752.4836 | contact@pharmacy.ohio.gov | www.pharmacy.ohio.gov (b) The dosage prescribed exceeds a daily average of eighty MED or at lower doses if the patient is co-prescribed a benzodiazepine, sedative hypnotic drug, carisprodol, tramadol, or gabapentin; or (c) The patient has a concurrent substance use disorder. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to submit the specified dispensing, personal furnishing, or wholesale sale information on all products containing gabapentin to the Ohio Automated Rx Reporting System (OARRS): As used in Chapter 4731-11 of the Administrative Code: (A) "Controlled substance" means a drug, compound, mixture, preparation, or substance included in schedule I, II, III, IV, or V pursuant to the provisions of Chapter 3719. of the Revised Code and Chapter 4729:9-1 of the Administrative Code. C.S.A. Controlled Substance Act *These are drug products which: (1) may be dispensed only upon a prescription issued by a practitioner and, (2) contain controlled substances but have been specifically excepted from the controlled substances schedules. (Title 21, CFR 1308.31.) Accordingly, these drugs are legally SHORT-ACTING **Ohio law requires prescribers to request and review an OARRS report before initially prescribing or personally furnishing any controlled substance, such as an opioid analgesic or a benzodiazepine, and gabapentin** LENGTHc OF AUTHORIZATIONS: Initial short-acting and long-acting requests may only be authorized for up to 90 days. (10) Authorize refills for schedules III and IV controlled substances only as permitted by section 3719.05 of the Revised Code. (11) Not authorize a refill beyond one year from the date of issuance for schedule V controlled substances and for dangerous drugs that are not controlled substances.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 | |
 |  |
 |  |
 |  |