Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
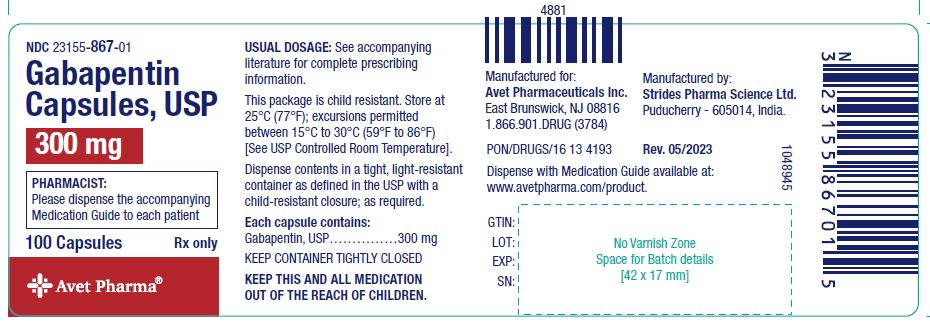 |  |
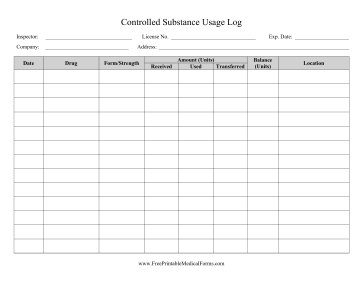
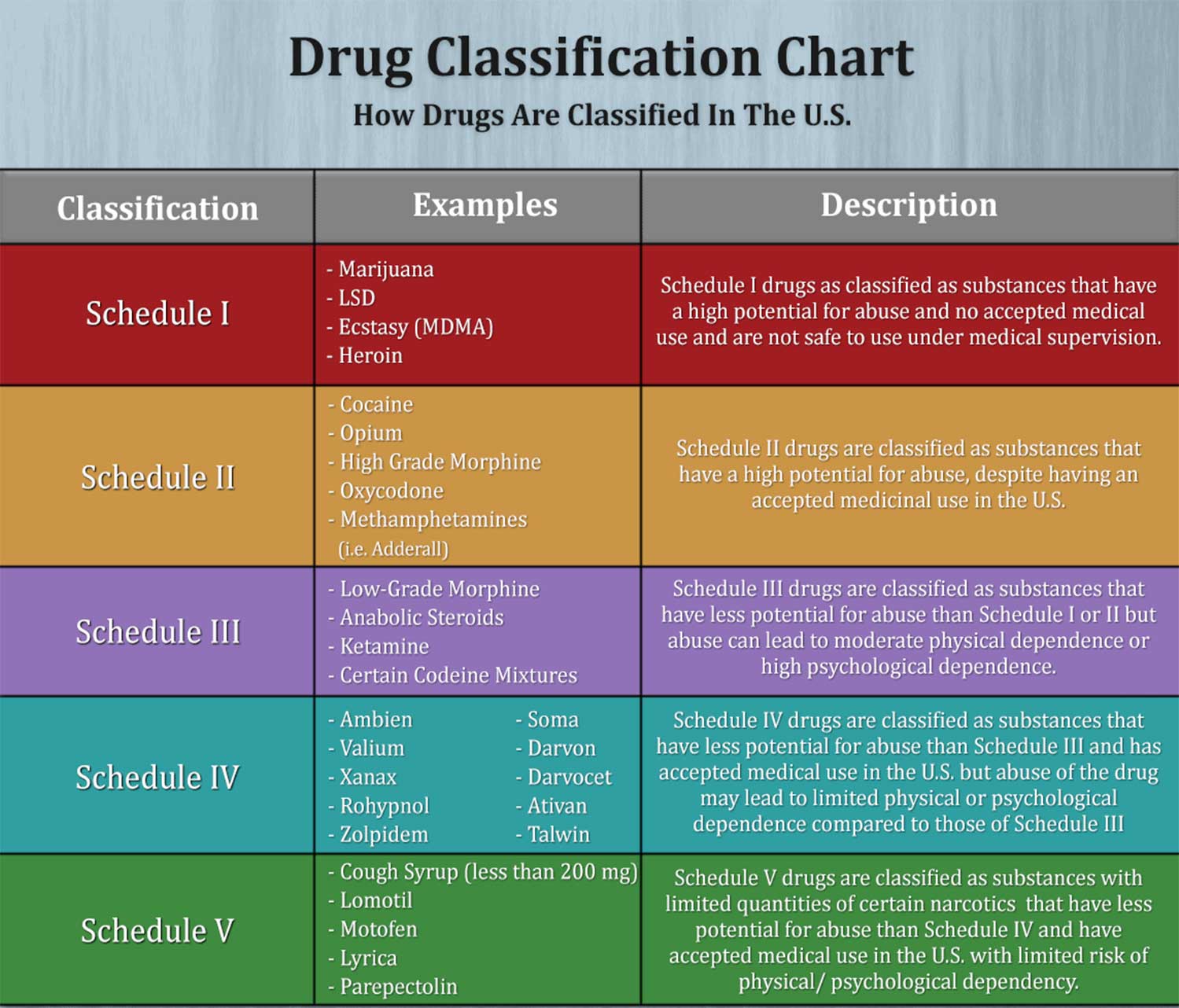
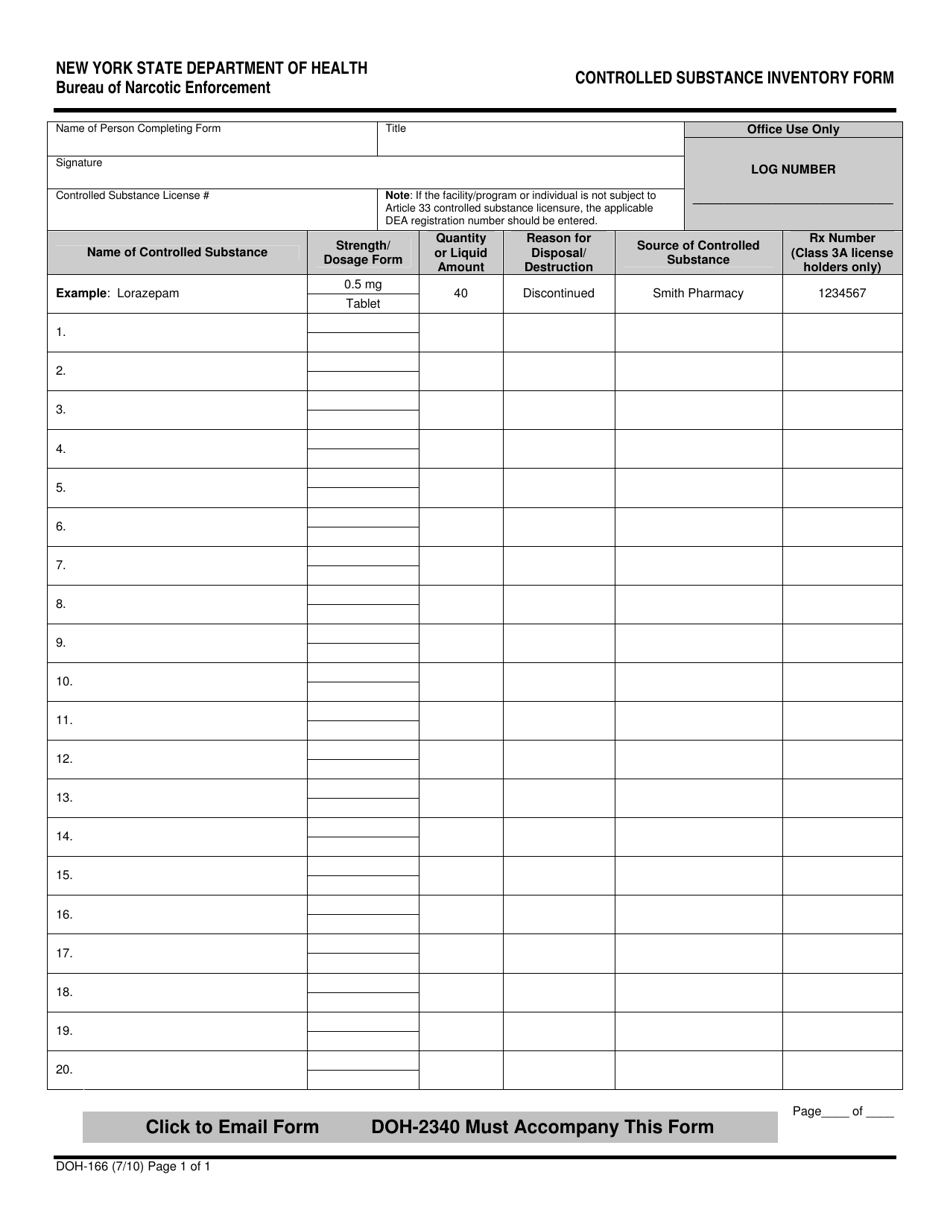
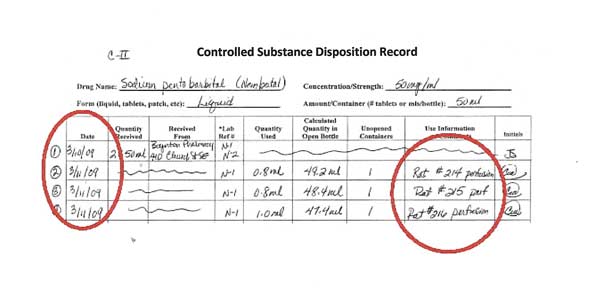
This article shall be known as the New York State Controlled Substances Act. § 3300-a. Legislative purposes. The purposes of this article are: 1. to combat illegal use of and trade in controlled substances; and 2. to allow legitimate use of controlled substances in health care, including palliative care; The prevalence of gabapentin abuse has been estimated to be 1.6% in the general population compared with 15% to 22% in patients with opioid use disorders. 23 Individuals with a history of substance abuse (particularly opioids) or mental illness have been identified as having an increased risk of abusing gabapentin. 23 Evidence of gabapentin abuse was observed in a group of 503 individuals in schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic JUSTIFICATION: Gabapentin (Neurontin) is a popular prescription drug that is approved by the U.S. Food and Drug Administration (FDA) for nerve pain and epilepsy treatment. However, through its mechanism of action, the drug boosts the effects of opioids to increase the euphoric effect. § 3306. Schedules of controlled substances. There are hereby established five schedules of controlled substances, to be known as schedules I, II, III, IV and V respectively. Such schedules shall consist of the following substances by whatever name or chemical designation known: Schedule I. (a) Schedule I shall consist of the drugs and other Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). 80.71 Practitioners; dispensing controlled substances 71 80.72 Issuance of official New York State prescription forms 73 80.73 Pharmacists; dispensing schedule II substances and certain other controlled substances 74 80.74 . Pharmacists; dispensing schedule III, IV and V controlled substances 78 Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. New York S3906 2019-2020 Classifies gabapentin as a controlled substance This bill reclassifies the drug gabapentin as a controlled substance in New York. It amends the public health law to add gabapentin to Schedule VI, which will require it to be monitored through the state's prescription monitoring program. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Subdivision 2 of section 3371 of the public health law is amended by adding a new paragraph (d) to read as follows: (D) FOR PURPOSES OF THIS SECTION, GABAPENTIN SHALL BE DEEMED TO BE A CONTROLLED SUBSTANCE. Establishes a schedule VI of drugs and other substances; includes gabapentin as a drug to be monitored through the prescription monitoring program; makes conforming changes; and directs the commissioner of health to promulgate regulations necessary or desirable to classify gabapentin and its chemical equivalents as a scheduled substance for the Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Adds gabapentin to the prescription monitoring program. S T A T E O F N E W Y O R K _____ 3823 2019-2020 Regular Sessions I N A S S E M B L Y January 31, 2019 _____ Introduced by M. of A. McDONALD, D'URSO, ENGLEBRIGHT, RIVERA, TAYLOR, SEAWRIGHT, McDONOUGH, MORINELLO, LAWRENCE, MONTESANO, BLANKENBUSH, BRAUNSTEIN, WILLIAMS, ARROYO, GOTTFRIED, JAFFEE, BLAKE -- Multi-Spon- sored by -- M. of A Subdivision 1 of section 3343-a of the public health law is amended by adding a new paragraph (e) to read as follows: (E) FOR PURPOSES OF THIS SECTION, GABAPENTIN (NEURONTIN, GRALISE, HORIZANT, GABARONE) AND ITS CHEMICAL EQUIVALENTS SHALL BE DEEMED TO BE A CONTROLLED SUBSTANCE. In an opposition memo to the now-defunct New York bill, the Drug Policy Alliance pushed back on the categorization of gabapentin as a controlled substance, arguing the medicine is “an effective Electronic Prescribing and Dispensing of Controlled Substances is now permissible in New York State Effective March 27, 2013 – Updated April 2013. Amendments to Title 10 NYCRR Part 80 Rules and Regulations on Controlled Substances have been adopted and became effective as final regulations on March 27, 2013. Prescribers licensed in New York to treat humans and who have a DEA registration number to prescribe controlled substances, as well as medical residents who prescribe controlled substances under a facility DEA registration number, must complete at least three (3) hours of course work or training in pain management, palliative care, and addiction. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |