Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
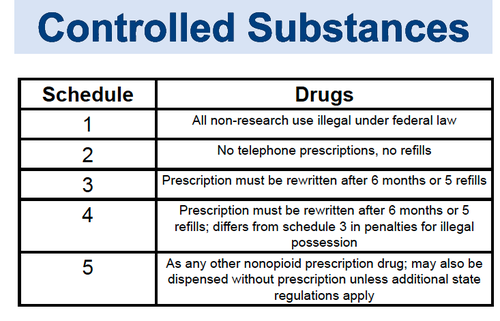
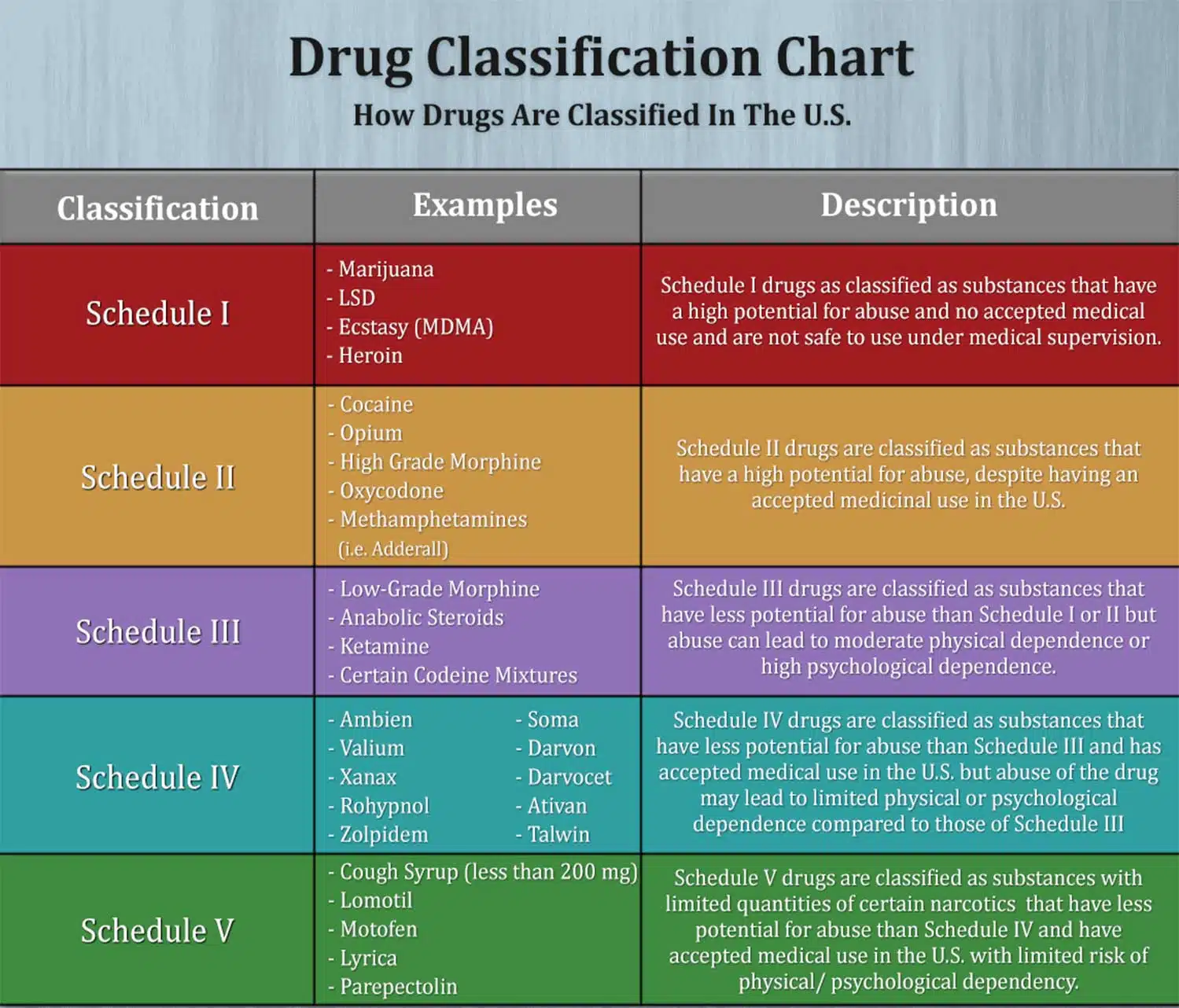
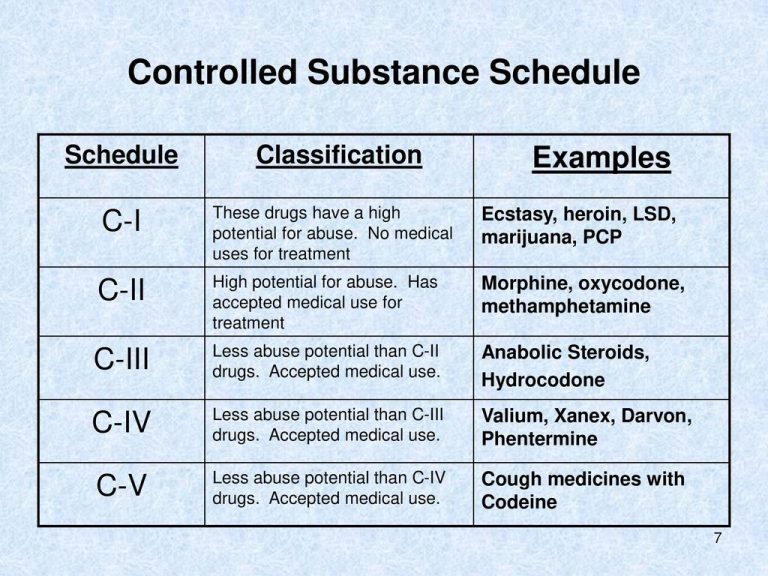
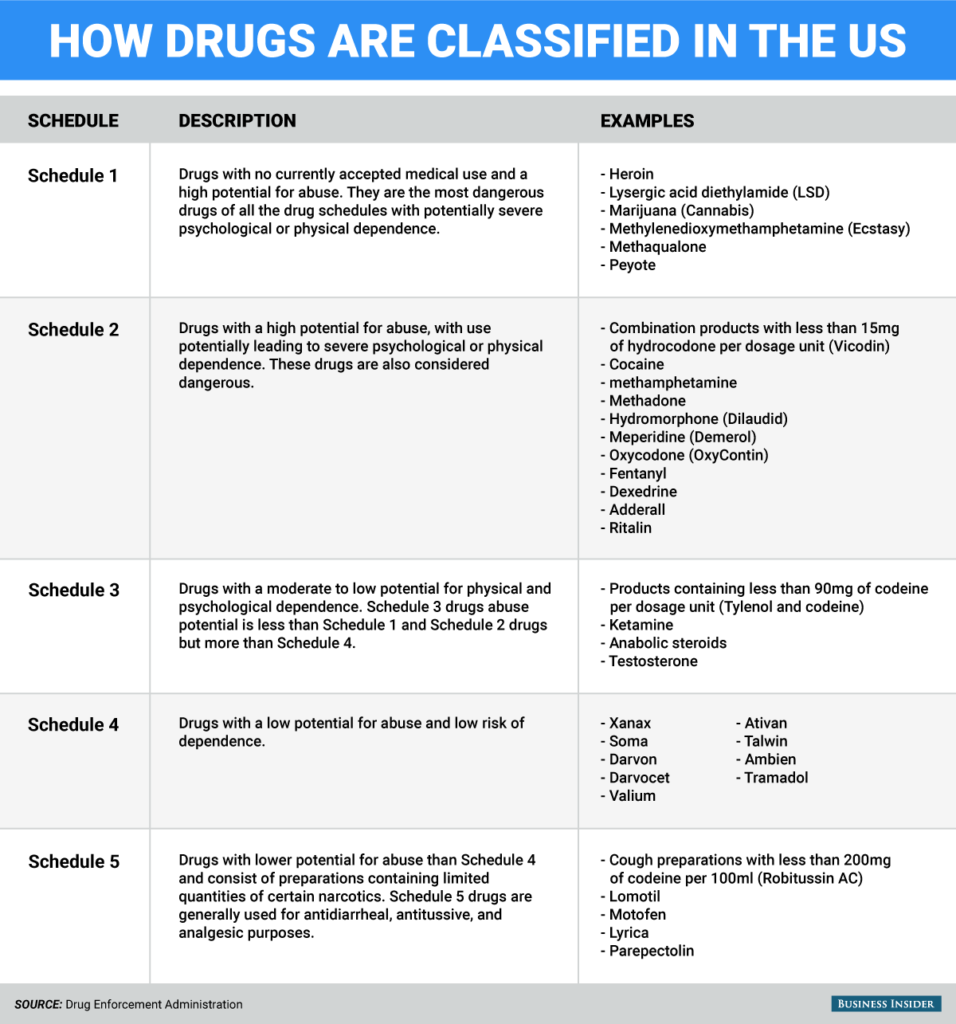
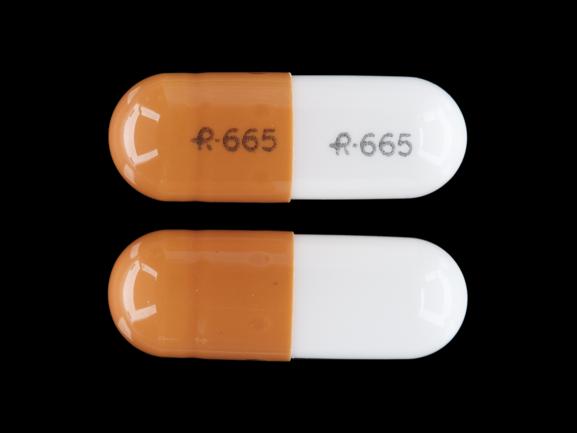
The Misuse of Drugs Act 1971 (Amendment) Order 2018 classifies pregabalin and gabapentin as Class C drugs under paragraph 1 (b) of Part 3 of Schedule 2 to the Misuse of Drugs Act 1971 (“the 1971 Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. The following drugs are listed as Schedule 3 (III) Drugs * by the Controlled Substances Act (CSA): The Controlled Substances Act (CSA) schedule information displayed applies to substances regulated under federal law. There may be variations in CSA schedules between individual states. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create While all other Schedule 3 CDs, including tramadol, pentazocine, the barbiturates, gabapentin, and pregabalin, as well as Schedule 2 drug quinalbarbitone are not subject to the same Safe Custody Regulations, it is an RCVS requirement that they are securely locked away. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. The reclassification of pregabalin and gabapentin to Schedule 3 of the Misuse of Drug Regulations 2001 from 1 April 2019 will affect vets. These schedule 3 drugs: will be exempt from purposes of this Schedule I hallucinogenic substances section only, the term “isomer” includes optical, position, and geometric isomers): (1) α-Ethyltryptamine (Other names: etryptamine; Monase; α- Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Gabapentin 3 28 days 7 7 7 7 Pregabalin 3 28 days 7 7 7 7 Further information PSNC has issued a briefing on gabapentin and pregabalin’s reclassification as Controlled Drugs, which includes a set of FAQs to outline what to do in certain scenarios: ow.ly/2oD030nKQpy NHS England has issued a briefing note on Rescheduling of Gabapentin and services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Background In January 2016, the Advisory Committee for the Misuse of Drugs recommended that pregabalin and gabapentin are scheduled as Sch 3 CDs within the Misuse of Drugs A. Schedule 2 and 3 CDs cannot be prescribed as part of the NHS repeat dispensing scheme. As of 1st April 2019, this means gabapentin and pregabalin will not be eligible for repeatable prescriptions. Q. Will pharmacies be remunerated for the Schedule 3 CD fee if prescriptions for gabapentin and pregabalin are Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin’s regulatory status varies by state. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. Gabapentin and pregabalin are in schedule 3, but not in the “must be kept locked in a CD cabinet” schedule 3 list. Hence, they do not need to be kept in the CD cabinet, recorded in the CD register or given with a witness. From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |