Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 | 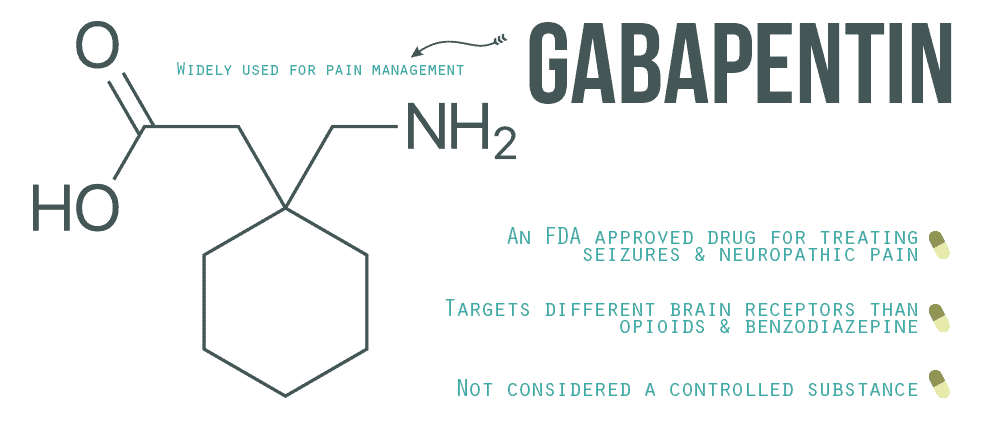 |
Columbus, OH— Gabapentinoids are prescribed primarily for off-label indications with limited evidence to support their benefits, but that has not stopped the steady increase in use of the drug class, according to a new study. Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Despite warnings that they are overprescribed for conditions they were never intended to treat, the use of gabapentinoids continues to grow in the United States. Pregabalin (Lyrica) and gabapentin (Neurontin) are both gabapentinoids, a class of nerve medication initially developed to treat epileptic seizures. GoodRx works to make its website accessible to all, including those with disabilities. If you are having difficulty accessing this website, please call or email us at 1-855-268-2822 or ada@goodrx.com so that we can provide you with the services you require through alternative means. Gabapentin enacarbil available under the trade name Horizant is the only gabapentin product approved for treatment of Restless Legs Syndrome (RLS). A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose, but caused an increase in adverse reactions. On January 25, 2024, Zydus launched AB-rated generic versions of Almatica’s Gralise (gabapentin) 300 mg and 600 mg tablets. — Gralise is also available as 450 mg, 750 mg and 900 mg tablets. Gralise is approved for the management of postherpetic neuralgia (PHN). 2025 Copyright | All Rights Reserved. Privacy Policy lyricaegj.com The most common form of gabapentin is a capsule containing powder, with the prescribed amount mixed with canned or soft food. The 100mg capsule is the most common size prescribed for cats. Gabapentin also comes in a 50mg/ml liquid form that does require refrigeration. The commercial liquid form may contain the artificial sweetener xylitol. Gabapentin is not currently scheduled at the federal level. While some states have reclassified gabapentin to Schedule V, others require that gabapentin prescriptions be reported to the prescription drug monitoring program (PDMP), and yet, other states have taken no action. Despite this warning, gabapentin prescribing has continued to increase in the United States. According to IQVIA National Prescription Audit, total prescriptions dispensed for gabapentin were approximately 68.3 million in 2019, 69.0 million in 2020, and 70.9 million in 2021. Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. If you have impaired kidney function, taking a lower dose or spacing out the dosing time is essential to prevent unwanted side effects.; Taking gabapentin with opioids (e.g., morphine, hydrocodone) can cause respiratory depression and sedation, and lead to fatal outcomes. Gabapentin is available as a generic; however, not all generics are interchangeable with some branded versions of gabapentin. Gabapentin may be used off-label (this means for an indication that has not been approved by the FDA but may still have a place in therapy) for some other indications such as fibromyalgia, persistent hiccups, migraine Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly "Gabapentinoid use continues to rise, increasingly co-administered with other sedatives, despite limited evidence to support their use and potential for harm," Goodman told MedPage Today. Gabapentin was first approved for use in the United Kingdom in 1993. [16] . It has been available as a generic medication in the United States since 2004. [17] . It is the first of several other drugs that are similar in structure and mechanism, called gabapentinoids. Experience a symphony of senses with Gabapentin, the breakthrough medication that can turn your world into a mesmerizing musical journey. Imagine hearing enchanting melodies, soul-stirring harmonies, and rhythmical beats Results can be sorted by generic name or latest revision date. Information related to affected product, reason for shortage, available products, estimated resupply dates, and alternative drug therapy is listed for each individual agent. Related: U.S. Drug Shortages: Root Causes and Recent Statistics. Current | Resolved | Discontinued Gabapentin is available as both a brand name product and a generic product (chemically the same, usually lower cost than the brand name product). Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. What is gabapentin approved for? Gabapentin is used to: Prevent and control partial seizures.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |