Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
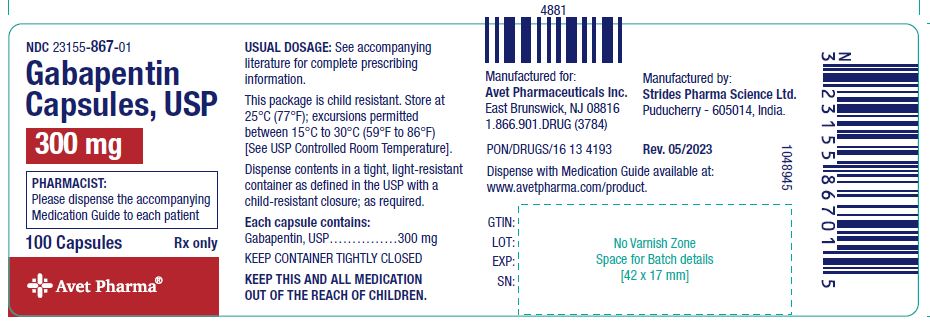 |
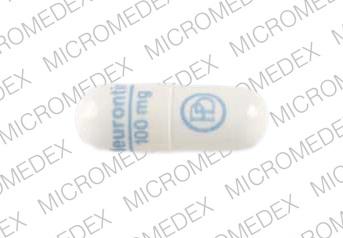
schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Indiana State law requires an ID to pick up a controlled substance. Pharmacies and other dispensers must collect an ID and record the identification number from anyone picking up a controlled substance. Records pertaining to controlled substances must be kept for 2 years. You can read about this in the federal code (link below). At this time gabapentin is not a federally-controlled substance. However, due to a spike in gabapentin-related fatalities, Ohio, Kentucky and West Virginia have moved to list the drug as a controlled substance at the state level. Although not classified under the federal Controlled Substances Act, the Indiana Board of Pharmacy designated gabapentin as a “drug of concern” in 2019. This designation signals the need for awareness without imposing the same restrictions as a controlled substance. Controlled Substances July 1, 2016: Pursuant to Indiana Code 25-26-13-4, ephedrine and pseudoephedrine, when dispensed via prescription, are each considered a "controlled substance" for the purposes of the INSPECT program. All prescription dispensations of these drugs must be reported to INSPECT. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. The practitioner must hold an Indiana Controlled Substance Registration ("CSR") and a federal Drug Enforcement Agency ("DEA") registration or is exempted from registration pursuant to 856 IAC 2-3-5(b) or 856 IAC 2-3-6 in order to prescribe a controlled substance. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Although gabapentin is not an opioid and is not designated a controlled substance by federal authorities, it can boost the high caused by narcotics, ward off drug withdrawals and block the effects As stipulated by IC 25-26-24-17, licensed dispensers throughout Indiana—and out-of-state (non-resident) pharmacies licensed to dispense drugs in Indiana—are required to submit controlled substance prescription data to INSPECT every twenty-four (24) hours. ATTENTION - Effective July 1, 2016, pursuant to IC 35-48-2-8(c)(3), Butalbital, commonly known as Fioricet, is a Schedule III substance in the State of Indiana.As a result, Schedule III requirements in the Indiana Controlled Substances Act and associated administrative rules in 856 IAC 2 apply to its handling and dispensation. As used in this chapter, "controlled substance" has the meaning set forth in IC 35-48-1-9. The term includes gabapentin. As added by P.L.246-2019, SEC.21 and P.L.264-2019, SEC.10. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. 2020 Indiana Code Title 25. "controlled substance" has the meaning set forth in IC 35-48-1-9. The term includes gabapentin. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored The prevalence of gabapentin abuse has been estimated to be 1.6% in the general population compared with 15% to 22% in patients with opioid use disorders. 23 Individuals with a history of substance abuse (particularly opioids) or mental illness have been identified as having an increased risk of abusing gabapentin. 23 Evidence of gabapentin abuse was observed in a group of 503 individuals in
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |