Gallery
Photos from events, contest for the best costume, videos from master classes.
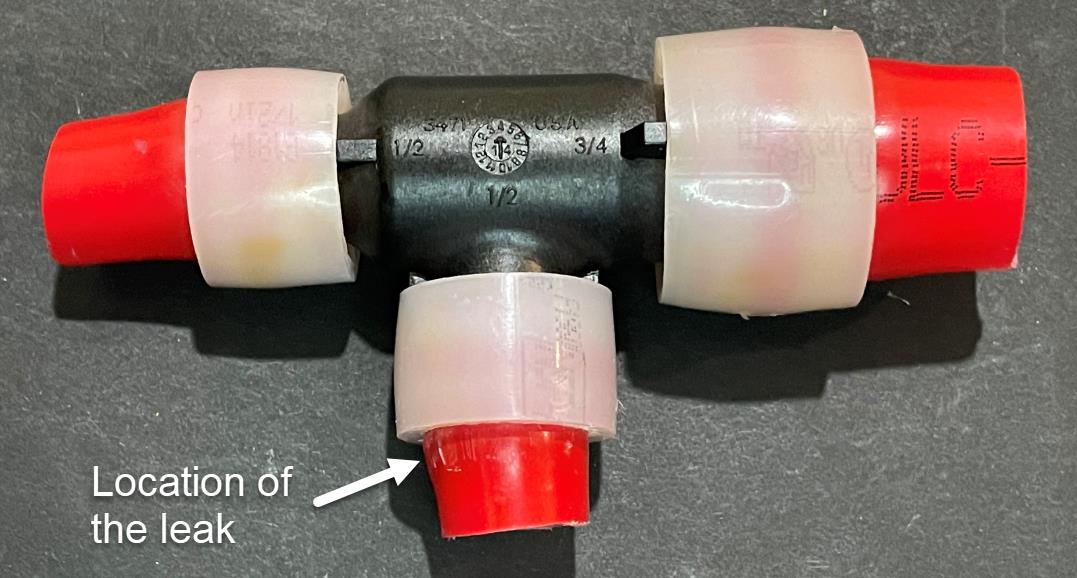 |  |
 |  |
 |  |
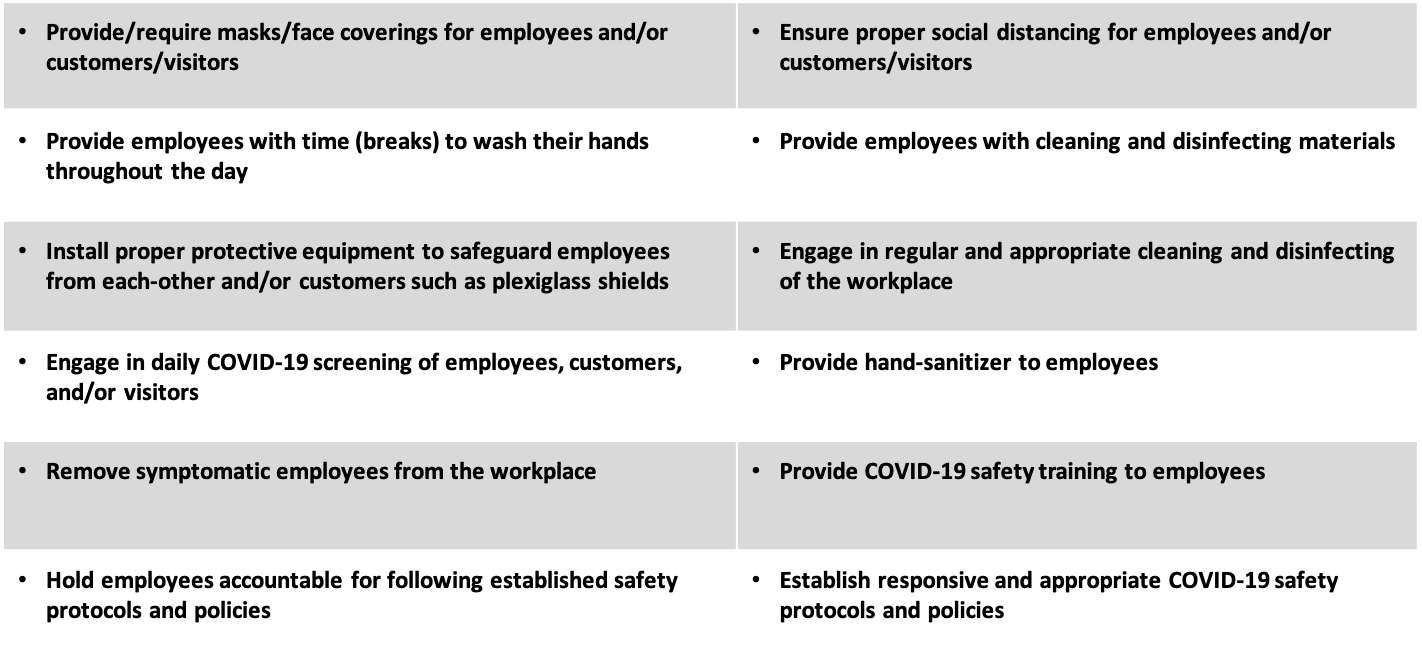 |  |
 |  |
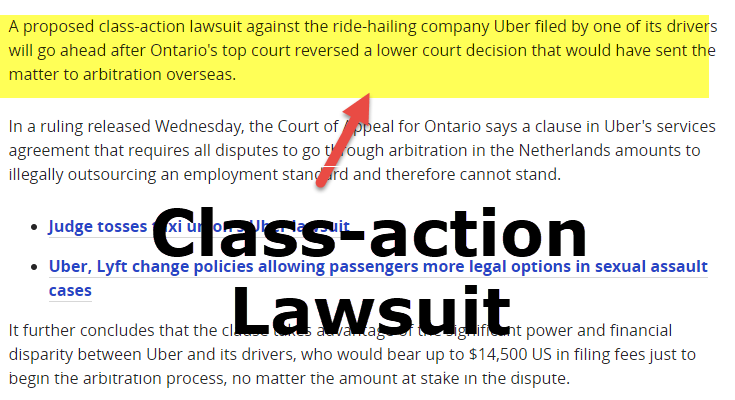 |  |
What Is Neurontin? Gabapentin, an anti-seizure drug marketed by Pfizer Laboratories in the United States under the trademark name Neurontin, has been the subject of numerous claims of malfeasance in gabapentin side effects lawsuits, with the Pfizer company intentionally and knowingly marketing the medication for uses not approved by the FDA (Food and Drug Administration). 6. Will I have to go to court for my Neurontin lawsuit? Many pharmaceutical cases settle before trial, but preparation for court proceedings is important. Your attorney will guide you through all necessary steps regardless of whether your case goes to trial. 7. Can I join a class action lawsuit for Neurontin-induced SJS? Discussion of the legal issues Nature of the Class Action: If you made at least one purchase of the brand name drug Neurontin directly from one of the defendants in this case and have also purchased generic gabapentin, your rights may be affected by a class action lawsuit, In re Gabapentin Lawsuit – How to Join. The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. To join the gabapentin lawsuit, potential class members need to meet the following criteria: New York, NY: A $190M settlement has been reached in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of Pfizer has reached a $190 million settlement agreement over claims that it manipulated the drug market to increase prices on its epilepsy drug Neurontin, resolving claims that the drug maker Gabapentin and pregabalin are members of a class of anti-convulsive and anti-epileptic drugs called gabapentinoids. Gabapentin was first approved in 1993 and pregabalin followed in 2004. They’ve been widely prescribed to treat certain types of pain as well. Conditions gabapentin and pregabalin are approved to treat include: A $190 million settlement has been reached in New York in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic versions of its epilepsy drug Neurontin. I oppose a class action suit against the manufacturers of gabapentin. At a personal level it helps me. At a medical level I have been reading gabapentin comments and experiences for several years and have found nothing that seems worse than other medications prescribed for nerve pain. I have seen nothing that rises to the level of a class MDL is not the same as class action, although an MDL can lead to a class-action lawsuit. A class action is a single lawsuit with several similar claimants. MDL cases remain separate lawsuits. The court does not consolidate MDL cases for a common outcome in the same way that class-action members share in the same settlement or verdict. One notable lawsuit emerged when the manufacturers of Neurontin reached a $325 million class action lawsuit settlement over allegations of fraudulent marketing practices. Pfizer Inc has agreed to pay $325 million to resolve claims it defrauded insurers and other healthcare benefit providers by marketing Neurontin for unapproved uses, its second settlement over In 2004, Pfizer agreed to pay $430 million in a DOJ settlement and pleaded guilty to two violations of the Food, Drug and Cosmetic Act for marketing the drug Neurontin, also known as gabapentin Brought by a class of third-party payers, the lawsuit alleges Pfizer and Warner-Lambert fraudently marketed Neurontin according to documents filed in Massachusetts federal court on Friday. Pfizer Inc. has agreed to a $190 million class action settlement that, if approved, would resolve claims that it delayed generic versions of its epilepsy drug Neurontin and promoted it for unapproved uses. The class action lawsuit was initially filed more than a decade ago by direct purchasers of Neurontin. The Neurontin settlement marks the end to a nearly 10-year-old class action lawsuit battle in which plaintiffs, who included both direct purchasers and third-party payers, accused Pfizer of delaying generic versions of Neurontin and promoted the drug for unapproved uses. 2. How much money did Pfizer pay in total for the gabapentin lawsuit? 3. Was there a class action lawsuit against Pfizer regarding gabapentin? 4. Is gabapentin a banned or controlled substance? 5. Why is there a new warning about gabapentin? 6. What are the most common side effects of gabapentin? 7. Can gabapentin cause addiction? 8. The active ingredient in Neurontin is gabapentin anhydrous. The lawsuit claimed that Pfizer delayed competition from less expensive generic versions of Neurontin by executing a multifaceted scheme involving, among other things, improperly listing certain patents with the U.S. Food and Drug Administration. engaging in illegal promotion and sales Yes, there have been significant lawsuits against manufacturers of gabapentin, primarily concerning the off-label marketing of the drug. While it’s not a singular, ongoing class-action lawsuit in the present day, the history of litigation surrounding gabapentin, particularly the brand name Neurontin, is extensive and
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |