Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
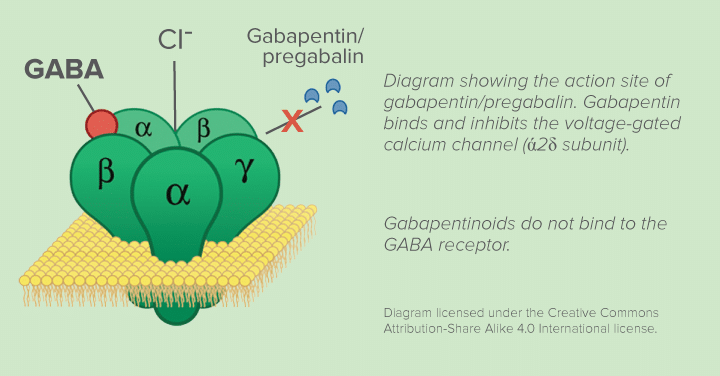
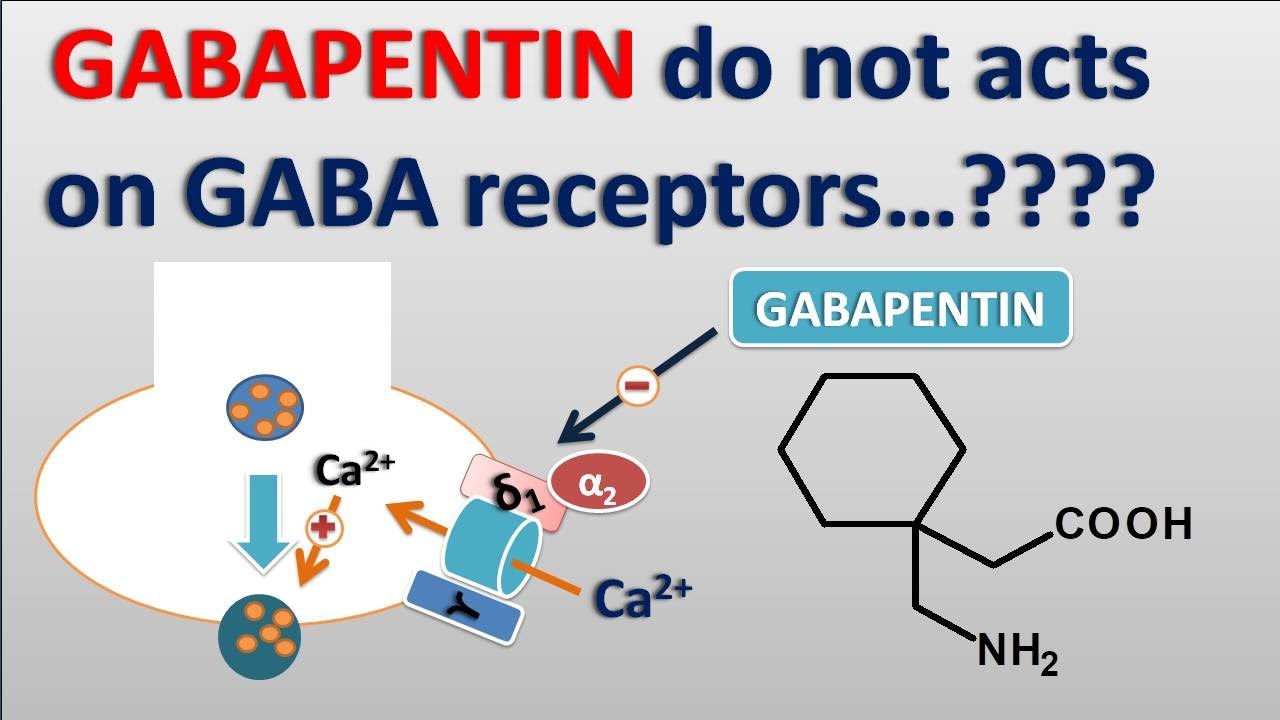
Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific bindin Pregabalin and gabapentin share a similar mechanism of action, inhibiting calcium influx and subsequent release of excitatory neurotransmitters; however, the compounds differ in their pharmacokinetic and pharmacodynamic characteristics. Gabapentin is absorbed slowly after oral administration, with m Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Pharmacokinetically, gabapentin has a unique absorption profile. It is absorbed in the small intestine via the L-amino acid transporter, and its bioavailability decreases with higher doses due to a saturable transport mechanism. Gabapentin is not significantly metabolized in the liver and is excreted unchanged via the kidneys. Gabapentin is a drug that modulates the release of excitatory neurotransmitters and is used for various indications. The half-life of gabapentin is 5 to 7 hours and it is not metabolized. Mechanism of action By inhibiting the voltage-gated calcium channels in the CNS, gabapentin reduces the release of excitatory neurotransmitters (mostly noradrenaline, dopamine and serotonin), and therefore decreases epileptogenesis. Gabapentin and pregabalin are structurally related compounds with recognized efficacy in the treatment of both epilepsy and neuropathic pain. The pharmacological mechanisms by which these agents exert their clinical effects have, until recently, remained unclear. The interaction of gabapentin and pr Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce different actions responsible for pain attenuation Herein we review the current understanding of the state‐dependent mechanisms of the gabapentinoids, the pathophysiological role of their molecular target, the α 2 δ calcium channel subunit, and the implications for clinical usage. Gabapentin crosses several lipid membrane barriers via system L amino acid transporters. In vitro, gabapentin modulates the action of the GABA synthetic enzyme, glutamic acid decarboxylase (GAD) and the glutamate synthesizing enzyme, branched-chain amino acid transaminase. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin binds preferentially to neurons in the outer layer of the rat cortex at sites that are distinct from other anticonvulsants Although the exact mechanism of action with the GABA receptors is unknown, researchers know that gabapentin freely passes the blood-brain barrier and acts on neurotransmitters. Gabapentin has a cyclohexyl group to the structure of the neurotransmitter GABA as a chemical structure. As with many other agents, GBP was licensed for the treatment of epilepsy with little or no understanding of its mechanism of action. Continued research and the parallel development of PGB have contributed to a contemporary pharmacological view of GBP (and PGB) as drugs with multiple modest cellular effects at therapeutic concentrations, but with a single predominant mechanism of action that Gabapentin is an anti-epileptic drug but its use has expanded to treat multiple other diseases including post-herpetic neuralgia, neuropathic pain, and spasticity. The mechanism of action is not fully understood but may be related to gabapentin’s action on calcium channels leading to diminution of excitatory neurotransmitters. Gabapentin misuse is increasing (oral, intranasal, and intravenous). Misuse can produce anxiolytic effects and a euphoria that is similar opioid misuse. Gabapentin can cause respiratory depression, physiologic dependence, and withdrawal symptoms on cessation (including diaphoresis, anxiety, confusion, and seizures). Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid ([]) that was first approved for use in the United States in 1993.It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures - today it is also widely used to treat neuropathic pain. Mechanism of action of gabapentinoids Site of action The actions of gabapentinoids are mainly at an intracellular site and require active uptake.21 They were originallydesigned as g aminobutyric acid (GABA) analogues but do not have any effects on GABA receptors. Gabapentin binds to a 2d receptors with greater affinity to the a 2d-1 subtype.22
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |