Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
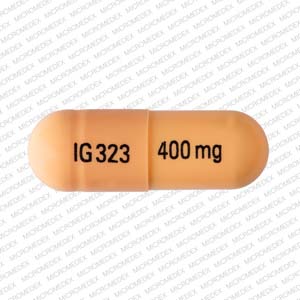
List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. In a single (400 mg) and multiple dose (400 mg three times a day) study of Gabapentin in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics. Manufacturers of Gabapentin that have been granted an NDC (National Drug Code). NDC: 49483-221-00 Code: 006T. Nitro-Time 6.5 mg ER Capsule 400 mg / White / Round. NDC: 49483-602-00. Gabapentin Capsules, USP. 400 mg / Orange, Orange NDC Package 74157-013-90 Gabarone Gabapentin Tablet Oral - View Billable Units, 11-Digit Format, RxNorm. RxCUI: 602407 - Gabarone 400 MG Oral Tablet; Product Type: Pill with imprint 400 mg IG323 is Orange, Capsule/Oblong and has been identified as Gabapentin 400 mg. It is supplied by InvaGen Pharmaceuticals, Inc. It is supplied by InvaGen Pharmaceuticals, Inc. Gabapentin is used in the treatment of Back Pain ; Postherpetic Neuralgia ; Epilepsy ; Chronic Pain ; Seizures and belongs to the drug class gamma In a single (400 mg) and multiple dose (400 mg three times a day) study of GABARONE in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics. Carbamazepine Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The product is distributed in 4 packages with NDC codes 65162-102-03, 65162-102-10, Search. Home; Lookup Tools. RxCUI: 310432 - gabapentin 400 MG Oral Capsule; 300 mg tablets: Gabapentin 300 mg tablets are white color, oval-shaped, film coated tablets debossed with “G5” on one side and “V1” on other side. NDC 31722-091-90 (Bottle of 90) 600 mg tablets: Gabapentin 600 mg tablets are yellow color, oval-shaped, film coated tablets debossed with “G7” on one side and “V1” on other side. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). Gabapentin is in a class of medications called anticonvulsants. The NDC Packaged Code 67877-223-05 is assigned to a package of 500 capsule in 1 bottle of Gabapentin, a human prescription drug labeled by Ascend Laboratories, Llc. The product's dosage form is capsule and is administered via oral form. Gabapentin extended-release tablets (Horizant) are used to treat restless legs syndrome (RLS; a condition that causes discomfort in the legs and a strong urge to move the legs, especially at night and when sitting or lying down). RxNorm is a normalized naming system for generic and branded drugs that assigns unique concept identifier(s) known as RxCUIs to NDC products.The NDC to RxNorm Crosswalk for this produdct indicates multiple concept unique identifiers (RXCUIs) are associated with this product: RxCUI: 476673 - gabapentin 100 MG Oral Tablet The product is distributed in 4 packages with NDC codes 45963-556-00, 45963-556-11, Search. Home; Lookup Tools. RxCUI: 310432 - gabapentin 400 MG Oral Capsule; Gabapentin records from the RxChat NDC Database. 0143-9994 Jul 09, 2007 Gabapentin 400 mg Oral Capsule by West-ward Pharmaceutical Corp. 0228-2636 Dec 14, Complete details for NDC 16571-0869-50 Gabapentin 400 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000140. gabapentin is a Oral Capsule in the Human Prescription Drug category. It is labeled and distributed by Sun Pharmaceutical Industries, Inc.. The primary component is Gabapentin. Sample Package? 62756-139 National Drug Code registration, ingredients, and packaging details. Find all the important details about this NDC Package code, including the 11-Digit NDC Billing number, billing units, wholesale price, RxNorm crosswalk, active ingredients, pharmacologic clasess, etc. Gabapentin is used with other medications to prevent and control seizures.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |