Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
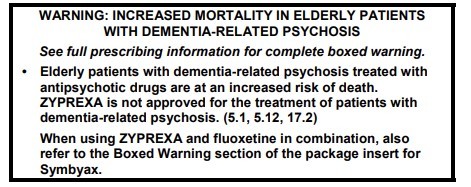
To address the serious risks of abuse, addiction, physical dependence, and withdrawal reactions, the U.S. Food and Drug Administration (FDA) is requiring the Boxed Warning be updated for all ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk Boxed warnings (formerly known as Black Box Warnings) are the highest safety-related warnings that medications can have assigned by the Food and Drug Administration. These warnings are intended to bring the consumer’s attention to the major risks of the drug. Although a black-box warning was not approved, the FDA collected data on the use of 11 anti-epileptic medications, including gabapentin, between 2005 and 2007 to determine whether there was indeed an increased risk of suicidal ideation or behavior. Medications affected by this warning include gabapentin (brand names: Neurontin and Gralise), gabapentin enacarbil (a gabapentin pro-drug under the brand name Horizant), pregabalin (brand names: Lyrica and Lyrica CR) and generic versions of gabapentinoids. The FDA issued a warning in December 2019 that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors.¹ The risk factors include the use of opioid analgesics and other drugs that depress the central nervous system (CNS) and These so-called “black box warnings,” given the border often found around them, are required by the U.S. Food and Drug Administration (FDA) for certain medications that carry serious safety risks. Black box warnings are the strictest labeling requirements that the FDA can mandate for prescription drugs. First implemented in 1979, black box warnings highlight serious and sometimes life-threatening adverse drug reactions within the labeling of prescription drug products. 1993 and pregabalin was first approved in 2004. Gabapentin is marketed under the brand names Neurontin and Gralise, and also as generics. Gabapentin enacarbil is marketed Abstract. The United States Food and Drug Administration issued a Black Box warning in October 2004 after placebo-controlled trials of antidepressant medications found an increased risk of suicidal thoughts and behaviors among children and adolescents taking antidepressant medications relative to placebo. The new warnings may lead people to file drug lawsuits over breathing-related injuries blamed on gabapentin’s and pregabalin’s respiratory risks. Poison control centers have reported increased calls about the gabapentinoids. The agency is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Among those factors are use of opioid pain medicines and other drugs that depress the central nervous system (CNS), as well as conditions such as Listen to an audio podcast of the December 19, 2019 FDA Drug Safety Communication warning that serious breathing difficulties may occur in patients using seizure and nerve pain medicines FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the Consumers and health professionals are advised that Boxed Warnings are being added to the Product Information (PI) and Consumer Medicine Information (CMI) for medicines containing pregabalin and gabapentin. The enhanced warnings advise that pregabalin poses a risk of misuse, while both pregabalin and gabapentin pose risks of abuse and dependence. The agency is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. A black box warning is the FDA’s most stringent warning for drugs and medical devices on the market. Black box warnings, or boxed warnings, alert the public and health care providers to serious side effects, such as injury or death. The FDA requires drug companies to add a warning label to medications that have a black box warning. Gabapentin is the sixth most prescribed drug in the USA. So the FDA wants everybody to take notice. The new gabapentin warnings are strong and direct. They result from incidences between 2012 and 2017, where the FDA said it received over 50 reports of "great concern". The reports described respiratory problems associated with using gabapentin A black box warning – often referred to as simply a “boxed warning” – is the strongest warning issued by the FDA in the United States on drugs that carry specific health risks – serious or life-threatening adverse effects.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |