Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
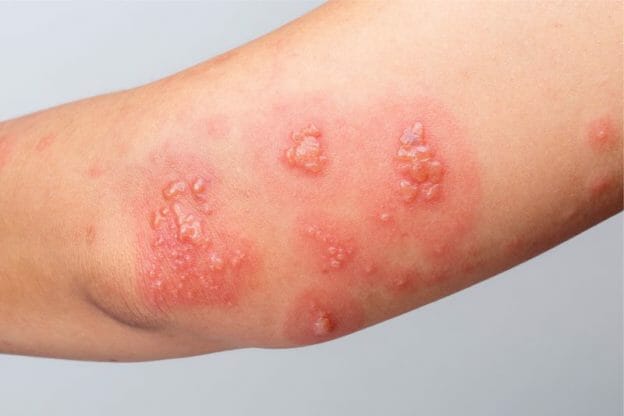
NEURONTIN (gabapentin) capsules, tablets, and oral solution are supplied as follows: 100 mg capsules: White hard gelatin capsules printed with "PD" on the body and "Neurontin/100 mg" on the Advise the patient to read the FDA-approved patient labeling (Medication Guide). Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity : Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times NEURONTIN is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS . 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels. We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels. The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- FDA approved labeling text (dated 10/12/00) Elimination: Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. NEURONTIN. NEURONTIN® (gabapentin) capsules,for oral use NEURONTIN® (gabapentin) tablets,for oral use NEURONTIN® (gabapentin) oral solution Initial U.S.Approval: 1993-----INDICATIONS AND USAGE-----NEURONTINis indicated for: Postherpetic neuralgia in adults (1) Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1,800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly 5.3 Withdrawal of Gabapentin 5.4 . Tumorigenic Potential 5.5 . Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity 5.6. Laboratory Tests 6 ADVERSE REACTIONS . 6.1 Clinical Trials Experience . 6.2 Postmarketing and Other Experience with other Formulations of Gabapentin . 7 DRUG INTERACTIONS . 7.1 Phenytoin NEURONTIN is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. 5 WARNINGS AND PRECAUTIONS 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan FDA Approved Labeling Text dated 03/01/2011 Page 2 . particular, gabapentin prevents pain-related responses in several models of neuropathic pain in rats or mice (e.g. spinal nerve ligation Drug Label Information. Updated November 5, 2010 If you are a consumer or patient please visit this version. Download DRUG LABEL INFO: PDF XML; Medication Guide: HTML; Official Label (Printer Friendly) Search for Labels on DailyMed. The labels are also available on the National Library of Medicine's DailyMed web site. You can search for labels by drug name and link to the Library’s For current labeling information, please visit particular, gabapentin prevents pain-related responses in several models of neuropathic pain in rats or mice Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |