Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 | |
 |  |
 | 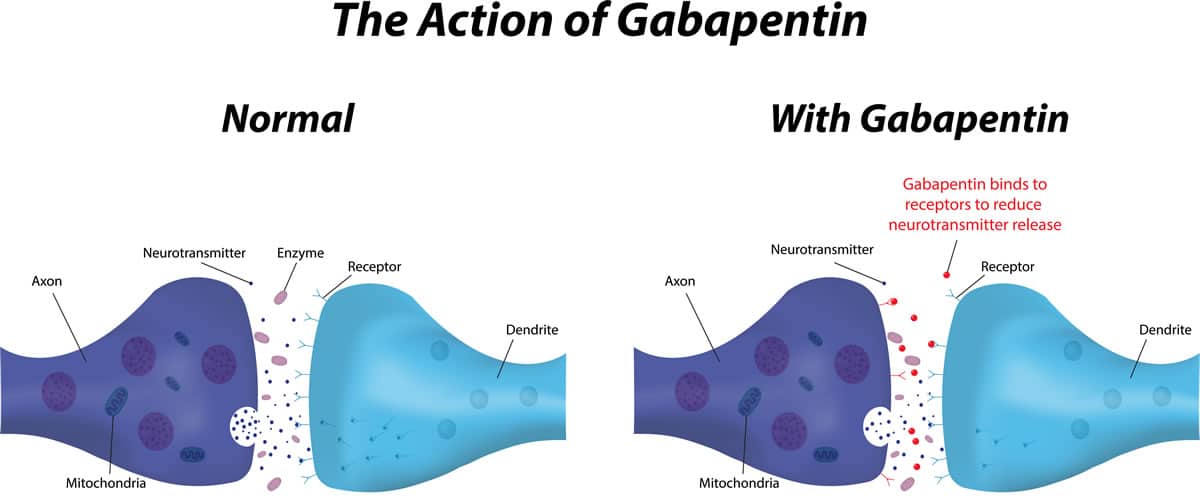 |
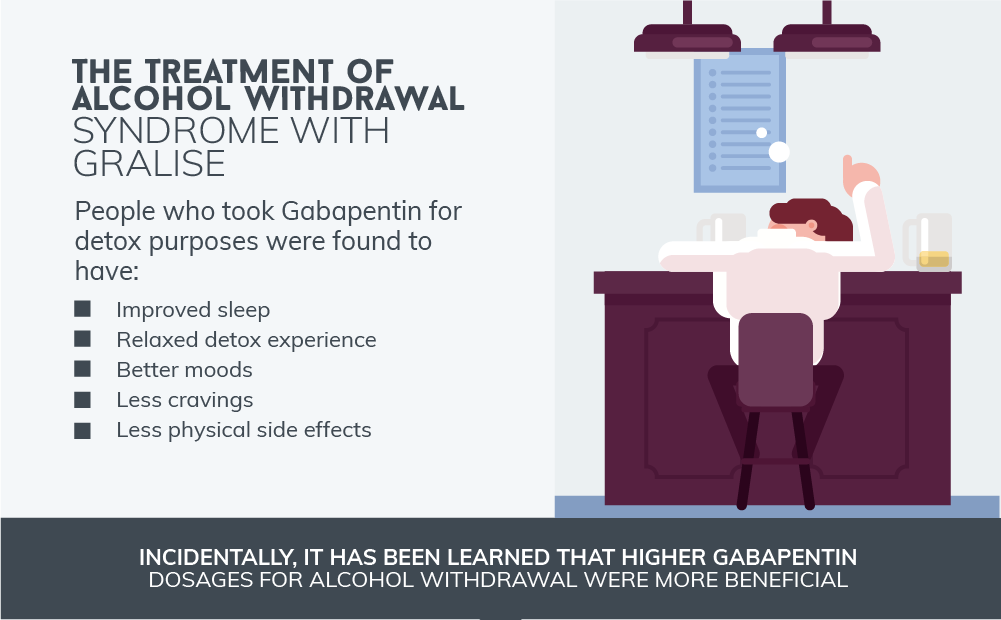
Pfizer Inc. has agreed to a $190 million class action settlement that, if approved, would resolve claims that it delayed generic versions of its epilepsy drug Neurontin and promoted it for unapproved uses. The class action lawsuit was initially filed more than a decade ago by direct purchasers of Neurontin. Another significant case occurred in 2010 when the company agreed to pay $325 million to settle a class-action lawsuit brought by third-party payers. The lawsuit alleged that Pfizer had deceptively marketed Neurontin, causing financial losses to the payers. If anyone knows of any kind of class action lawsuit or compensation for this drug please let me know my email address is sicstgrace2316@gmail.com or 444wolfe@gmail.com my name is Rachel Hutchins The Court has certified a class of direct purchasers of Neurontin and preliminarily approved a proposed Settlement of the Class Action. The Settlement provides for payment by Defendants of $190,000,000.00 (one hundred ninety million dollars) plus interest into an escrow account (the “Settlement Fund”). A federal appeals court has upheld a $142 million jury verdict in a case involving Pfizer’s epilepsy drug Neurontin, and opened the door for potentially even more lawsuits. Brought by a class of third-party payers, the lawsuit alleges Pfizer and Warner-Lambert fraudently marketed Neurontin according to documents filed in Massachusetts federal court on Friday. A $190 million settlement has been reached in New York in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic versions of its epilepsy drug Neurontin. The Lawsuit is known as In re Neurontin Antitrust L itigati on, Civil Action No. 02-1390. Judge Faith S. Hochberg of the United States District Court for the District of New Jersey is overseeing this class action. 3. What is a class action? In 2004, Parke-Davis, a division of Warner-Lambert that was acquired by Pfizer, paid $430 million to the U.S. Justice Department over claims that they were illegally promoting Neurontin for The active ingredient in Neurontin is gabapentin anhydrous. The lawsuit claimed that Pfizer delayed competition from less expensive generic versions of Neurontin by executing a multifaceted scheme involving, among other things, improperly listing certain patents with the U.S. Food and Drug Administration. engaging in illegal promotion and sales I take the medication called gabapentin 300 mg there’s a class action lawsuit against this medication it has some very disturbing side effects but I think it have infected me one of the side Neurontin class action settlement to compensate third-party payors who purchased, paid for, administered and/or reimbursed for gabapentin sold by Pfizer. Neurontin Lawsuit | 2025 Latest Updates The prescription pain reliever Neurontin (generic gabapentin) has recently been linked to an increased risk for Stevens-Johnson syndrome (SJS), a severe skin disorder in which the top layer of the skin dies, followed by a painful rash that spreads and blisters. A Diversified Practice. The Schmidt Firm, PLLC has focused its practice on the representation of plaintiffs involved in both traditional personal injury and wrongful death litigation – involving medical malpractice, transportation accidents and nursing home abuse – as well as mass tort and toxic tort litigation – involving defective or dangerous pharmaceuticals, medical devices and toxic The Neurontin settlement marks the end to a nearly 10-year-old class action lawsuit battle in which plaintiffs, who included both direct purchasers and third-party payers, accused Pfizer of delaying generic versions of Neurontin and promoted the drug for unapproved uses. Pfizer Inc has agreed to pay $325 million to resolve claims it defrauded insurers and other healthcare benefit providers by marketing Neurontin for unapproved uses, its second settlement over the Gabapentin's exact mechanism of action is not fully understood, but it is believed to work by reducing abnormal electrical activity in the brain. It is thought to bind to calcium channels, modulating their activity and reducing the release of neurotransmitters involved in seizures and nerve pain. What Is Neurontin? Gabapentin, an anti-seizure drug marketed by Pfizer Laboratories in the United States under the trademark name Neurontin, has been the subject of numerous claims of malfeasance in gabapentin side effects lawsuits, with the Pfizer company intentionally and knowingly marketing the medication for uses not approved by the FDA (Food and Drug Administration). Gabapentin Lawsuit – How to Join. The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. To join the gabapentin lawsuit, potential class members need to meet the following criteria: New York, NY: A $190M settlement has been reached in a consumer fraud class action lawsuit pending against Pfizer which alleges the pharma giant engaged in tactics to delay market entry of generic
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 | |
 |  |
 |  |