Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
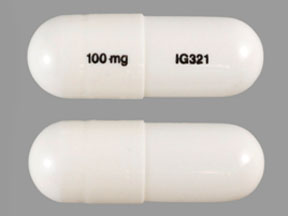
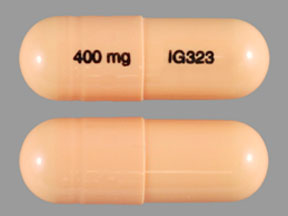
Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Although gabapentin isn’t a federally controlled substance, some states have classified it as one. These states consider gabapentin risky enough to warrant controlled substance status. Controlled substances are divided into five schedules, or groups, in total. Substances are grouped by their potential risk for misuse and dependence. January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly At the time of this writing, gabapentin (Neurontin) is not considered to be a controlled substance on the federal level. However, some states, such as Michigan, and other municipalities, have scheduled it as a class 5 drug, meaning it is believed to have a relatively low potential for abuse. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. While gabapentin is not considered a controlled substance and is not typically associated with the same level of addictive potential as opioids or benzodiazepines, it's important to be aware of the potential for dependence and misuse. Like any medication, gabapentin should be used as directed by your healthcare provider. Effective July 1, 2018, all gabapentin products will be Schedule V controlled substances in the state of Tennessee. It is known under the brand names Neurontin, Horizant, Gralise, Gabarone, and Fanatrex. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The federal government doesn't consider gabapentin (Neurontin) a controlled substance. However, gabapentin (Neurontin) has been shown to be potentually misused, such as causing a "high" when not used properly and when used at higher doses. Because of this risk, some states currently classify gabapentin (Neurontin) as a controlled substance Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970.11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored Gabapentin is not a federally-controlled drug substance and does not contain an opioid (narcotic) medication. However, gabapentin misuse and abuse has been reported, and it may be restricted in some states through their state drug-monitoring program. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. While gabapentin remains a non-controlled substance, Session Law 2023-65 Part XI Section 11.1 G.S. 90-113.73(b) adds it to the medications recorded in NC CSRS because it may cause a level of sedation in patients that puts them at increased risk of overdose when taken with opioids. The NC Department of Health and Human Services (NCDHHS) has
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |