Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
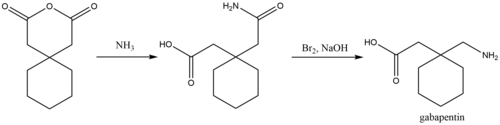
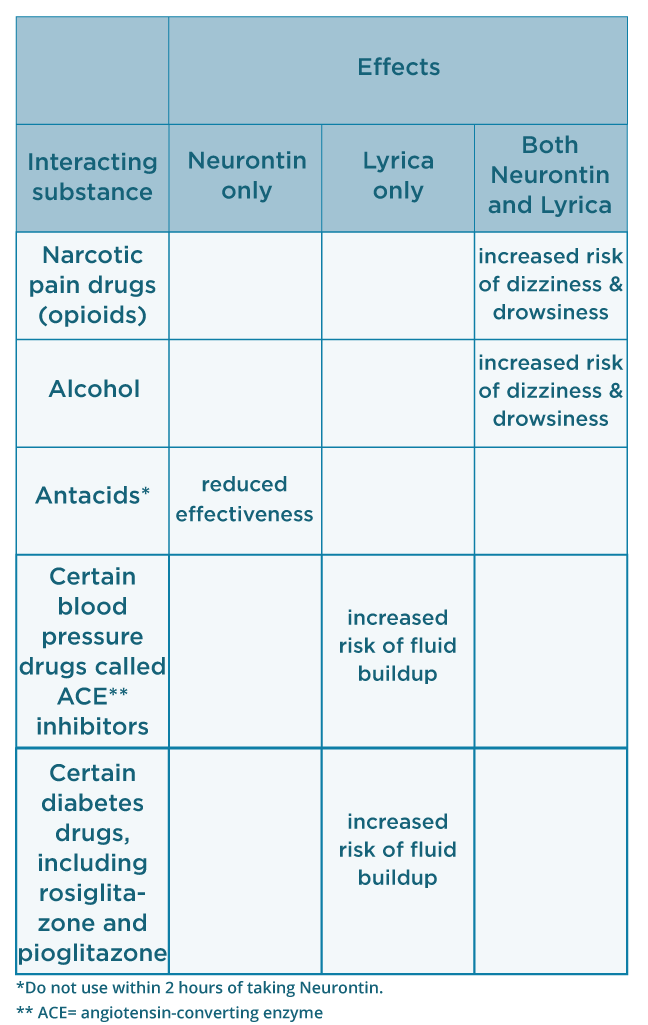
The NADAC wholesale price of Neurontin 0071-0806-40 has fluctuated from a low of $2.85999 to a high of $6.76530 per billable unit over a period of 5 years and The historical price table below includes all the price changes based on the monthly surveys of retail and chain pharmacies taken by the Centers for Medicare and Medicaid Services (CMS). Gabapentin, under the brand name Neurontin, was first approved in May 1993 for the treatment of epilepsy in the United Kingdom, and was marketed in the United States in 1994. [43] [44] Subsequently, gabapentin was approved in the United States for the treatment of postherpetic neuralgia in May 2002. [45] First developed in the 1970s, gabapentin was approved by the FDA in 1993 for use in the United States. It has been marketed under the name Neurontin, although other brand names exist. Gabapentin is structurally related to gamma-aminobutyric acid (GABA), a neurotransmitter in the brain. When prescribing gabapentin, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8,10 Gabapentin has Neurontin/Gabapentin comes as a capsule to take by mouth. Neurontin is taken three times a day. To minimize Neurontin side effects, take the very first Neurontin dose at bedtime. Then take this Neurontin medication at evenly spaced times throughout the day and night to ensure a constant level of Neurontin/gabapentin in your body. Never take double doses of Neurontin (gabapentin) that’s an adamant rule whereas on Neurontin (gabapentin) medication. However, if you missed a dose on that point and promptly remembered it hours before consequent dose, you will take one to catch up. however if it’s close to the time for consequent dose, higher forego that missed dose and proceed to consequent one. Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. Recently, lawsuits alleging damages from illegal marketing of another old drug, gabapentin (Neurontin), have yielded remarkable discoveries about the structure and function of pharmaceutical When prescribing NEURONTIN, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Gabapentin is an anticonvulsive medication that received approval from the US Food and Drug Administration (FDA) in 1993 and has been available in generic form in the USA since 2004. Gabapentin was originally used as a muscle relaxant and an anti-spasmodic. However, it was later discovered that gaba Accordingly, the goal of this review is to examine the history of gabapentin, relevant data on efficacy across nonapproved indications, and ethical considerations that should be considered regarding its use, to assist health care providers in applying a more stringent assessment of the risk-benefit balance of prescribing gabapentin for various “The history of gabapentin is really a history of uses getting ahead of the evidence,” said Dr. Joseph Ross, an internist and health policy researcher at Yale School of Medicine. When prescribing NEURONTIN, carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g., self-dose escalation and drug-seeking behavior). Gabapentin, a GABA receptor agonist, was first studied as an antiepileptic drug in humans in 1987 (07). It was launched in the United Kingdom in 1993 and approved in the United States as add-on therapy for intractable partial seizures in adults. It is also approved for the treatment of postherpetic neuralgia. Gabapentin was originally discovered over 40 years ago by the Japanese, who initially were looking for an antispasmodic or muscle relaxant. It was later sold to Parke-Davis (Warner-Lambert, which merged with Pfizer in 2000), who discovered effectiveness of gabapentin for treating epileptics. The only contraindication for both gabapentin and pregabalin is in patients who have demonstrated hypersensitivity to either drug/ingredients. Gabapentin has 2 drug-drug interactions listed in its most recent product insert, with the mechanisms not known: Coadministration of gabapentin with hydrocodone decreases hydrocodone exposure. A chemical synthesis of gabapentin has been described. History. Gabapentin was developed at Parke-Davis and was first described in 1975. Under the brand name Neurontin, it was first approved in May 1993, for the treatment of epilepsy in the United Kingdom, and was marketed in the United States in 1994. The case did little to stop the march of Neurontin. By 2002 the treatment of neuropathic pain had been approved both in the United States and the UK. Then, in 2004, Pfizer released pregabalin, Gabapentin was originally marketed under the brand name Neurontin. Since it became generic, it has been marketed worldwide using over 300 different brand names. [ 1 ] An extended-release formulation of gabapentin for once-daily administration was introduced in 2011, for postherpetic neuralgia under the brand name Gralise.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |