Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
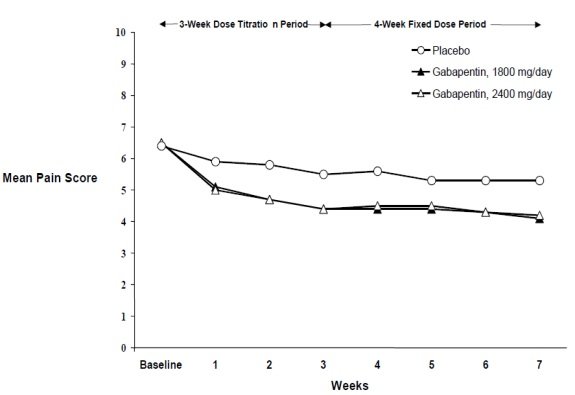
GABAPENTIN- gabapentin tablet, film coated GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg NEURONTIN capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/ day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Neurontin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES. GABAPENTIN capsules, for oral use Initial U.S. Approval: 1993----- RECENT MAJOR CHANGES -----Warnings and Precautions, Respiratory Depression (5.7) consider initiating gabapentin at a low dose. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. DOSAGE AND USE See package insert for full prescribing information. Each capsule contains 100 mg of gabapentin. NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50mg/kg/day have been well tolerated in a NEURONTIN (gabapentin) is indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. 1.1 Pediatrics 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three See package insert . for full prescribing information. STORAGE AND HANDLING. Store at 20 to 25 C (68 to 77 F); excursions GABAPENTIN (UNII: 6CW7F3G59X GABAPENTIN- gabapentin solution Amneal Pharmaceuticals LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN ORAL SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 INDICATIONS Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Gabapentin oral solution is indicated for: • Postherpetic neuralgia in adults ( 1) • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy ( 1) Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. Pediatric Patients Age 3 to 11 years.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |