Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
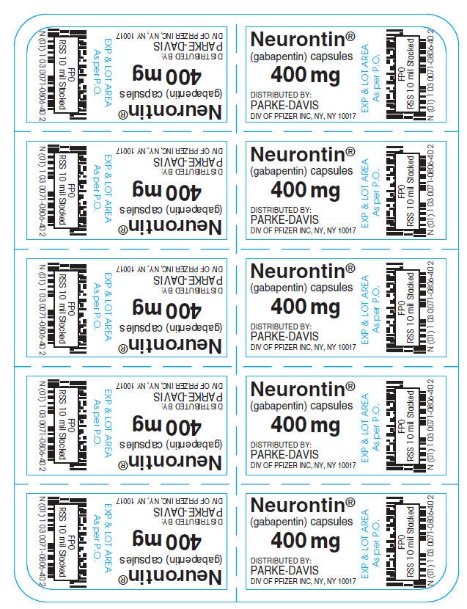
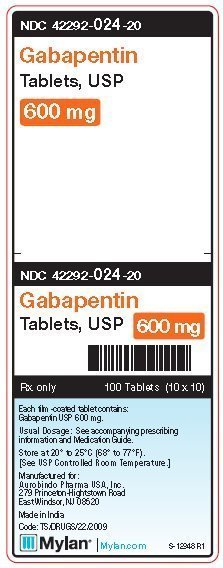
NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® is indicated for: • Management of postherpetic neuralgia in adults - • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50mg/kg/day have been well tolerated in a Highlights of Prescribing Information. T hese highlights do not include all the information needed to use gabapentin capsules safely and. effectively. See full prescribing information for gabapentin capsules. GABAPENTIN capsules, USP for oral use. Initial U.S. Approval: 1993 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN® (gabapentin) capsules, for oral use NEURONTIN® (gabapentin) tablets, for oral use NEURONTIN® (gabapentin) oral solution Initial U.S. Approval: 1993 1 FULL PRESCRIBING INFORMATION 2 . GRA-004-C.5 SEP 2012 3 GRALISE® (gabapentin) Tablets 4 . 1 INDICATIONS AND USAGE 5 . GRALISE is indicated for the management of postherpetic neuralgia. 6 GRALISE is not interchangeable with other gabapentin products because of differing 7 . pharmacokinetic profiles that affect the frequency of administration. 8 Formulation/presentation: Neurontin ® (Gabapentin) capsule is available in 100 mg × 50’s and 300 mg × 100’s. The tablet is available in 600 mg × 100’s. Indications: Adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children >3 yrs. HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 TABLE 1. NEURONTIN Dosage Based on Renal Function; TID = Three times a day; BID = Two times a day; QD = Single daily dose * For patients with creatinine clearance <15 mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patients with a creatinine clearance of 7.5 mL/min should receive one-half the daily dose that patients with a creatinine clearance of 15 mL/min receive). FULL PRESCRIBING INFORMATION: CONTENTS* HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GRALISE safely and effectively. See full prescribing information for GRALISE. GRALISE ® (gabapentin) tablets, for oral use Initial U.S. Approval: 1993 ----- Warnings and Precautions (5.2) 4/2020 The U.S. Physician Prescribing Information and Patient Information on this page may no longer be current. Please visit Viatris.com for the most current product information. for Consumers: NEURONTIN U.S. Medication Guide; for Healthcare professionals: NEURONTIN U.S. Physician Prescribing Information HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. (5.1) NEURONTIN ® (gabapentin) capsules, for oral use . NEURONTIN ® (gabapentin) tablets, for oral use . NEURONTIN ® (gabapentin) oral solution . Initial U.S NEURONTIN ® is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. 2. Neurontin Dosage and Administration. Gabapentin is indicated for: • Postherpetic neuralgia in adults (1) • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy (1) provided effective treatment in painful diabetic neuropathy in a randomized, double-blind study compared to placebo. provided significant reduction in weekly mean pain score in patients with painful diabetic neuropathy in a randomized, double-blind study versus placebo. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Neurontin is an anti-epileptic drug, also called an anticonvulsant. It affects chemicals and nerves in the body that are involved in the cause of seizures and some types of pain. Neurontin is used in adults to treat neuropathic pain (nerve pain) caused by herpes virus or shingles (herpes zoster). In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |