Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
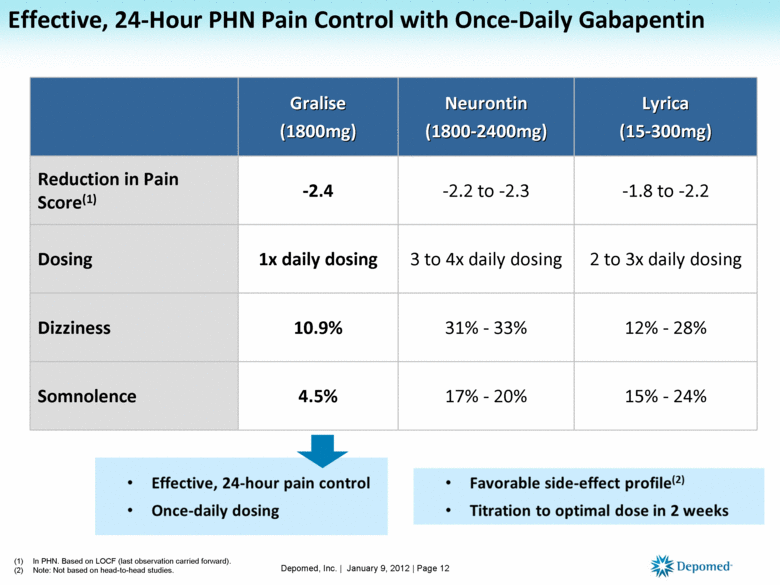
Gabapentin is a prescription medication approved by the Food and Drug Administration (FDA) for the treatment of neuropathic pain and epileptic disorders. It is currently marketed in capsule, tablet and oral solution formulations. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. 2.3 Dosage Adjustment in Patients with Renal Impairment . 2.4 Dosage in Elderly 2.5 Administration Information . 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS . 5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity . 5.2 Anaphylaxis and Angioedema The General Assembly's Illinois Administrative Code database includes only those rulemakings that have been permanently adopted. This menu will point out the Sections on which an emergency rule (valid for a maximum of 150 days, usually until replaced by a permanent rulemaking) exists. Day 2: 300 mg orally 2 times day Day 3: 300 mg orally 3 times a day. Titrate dose as needed for pain relief; Maintenance dose: 900 to 1800 mg/day orally in 3 divided doses Maximum dose: 1800 mg per day Extended-release: Gralise (gabapentin) 24-hour extended-release tablets: Initial dose: Day 1: 300 mg orally with the evening meal PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Always verify the schedule of combination products. Contact pharmacist if there is a question. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Schedule II/IIN Controlled Substances (2/2N) Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence. Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl Schedule 2 (II) Drugs. The drug has a high potential for abuse. The drug has a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions. Abuse of the drug may lead to severe psychological or physical dependence. • Schedule V drugs have an even lower potential for abuse, but may include a limited quantity of narcotics. Examples include some cough medicines (Robitussin AC) and Lyrica. WHAT DRUGS DO PDMPs MONITOR? PDMPs track Schedule II, III and IV drugs in every state, and Schedule V drugs in 33 states and D.C., including Pennsylvania. For schedules, the rule changes adopt the federal schedule subject to drugs scheduled by the state after January 6, 2022, and the rules promulgated by the Michigan Board of Pharmacy; remove Brorphine, Gabapentin, and Pentazocine as exceptions to the federal schedule; provide an exception to the federal scheduling for isomers, Salvia Divorum 95% of patients receive gabapentin for off-label indications.2 The off-label indications from this review include pain, bipo-lar affectivedisorder, restless leg syndrome, multiple sclerosis, anxiety, and other behavioral disorders.2 Gabapentin mayalso be used off label for alcohol use disorder, alcohol withdrawal, 2[\frgrqh ,, 2[\ ,5 2[\&rqwlq 2[d\gr 5r[lerqg 5r[lfrgrqh ;wdps]d (5 12 krxuv rqo\ 2[\frgrqh dfhwdplqrskhq frper ,, (qgrfhw 3hufrfhw 3ulpohy 3urodwh 5r[lfhw ;duwhplv ;5 12 krxuv rqo\ 2[\frgrqh dvslulq frper ,, (qgrgdq 3hufrgdq 12 krxuv rqo\ 2[\frgrqh qdor[rqh frpelqdwlrqv ,, 7dujlqlt (5 7ur[\fd (5 12 krxuv rqo\ Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. Neurontin is used in adults to treat neuropathic pain (nerve pain) caused by herpes virus or shingles (herpes zoster). Neurontin is also used to treat seizures in adults and children who are at least 3 years old. Use only the brand and form of gabapentin your doctor has prescribed. Schedule II drugs, substances, or chemicals are defined as drugs with a high potential for abuse, with use potentially leading to severe psychological or physical dependence. These drugs are also considered dangerous. gabapentin (Scheduled V in KY, not scheduled federally) barbital, methylphenobarbital and phenobarbital (Schedule III in KY, Schedule IV federally) --Be advised--KY does not exempt ANY butalbital product from the Control Substance Act (902 KAR 55:045) No Schedule I substances; Up to a 3-day supply of a Schedule II narcotic; non-narcotic Schedule II’s may be prescribed just like a physician; No other limitations other than the Opioid Reduction Act (Can be found in the Pharmacy Law Book) Lost or Stolen Controlled Substances. As required by § 15-2-9.3.1:
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |