Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 | 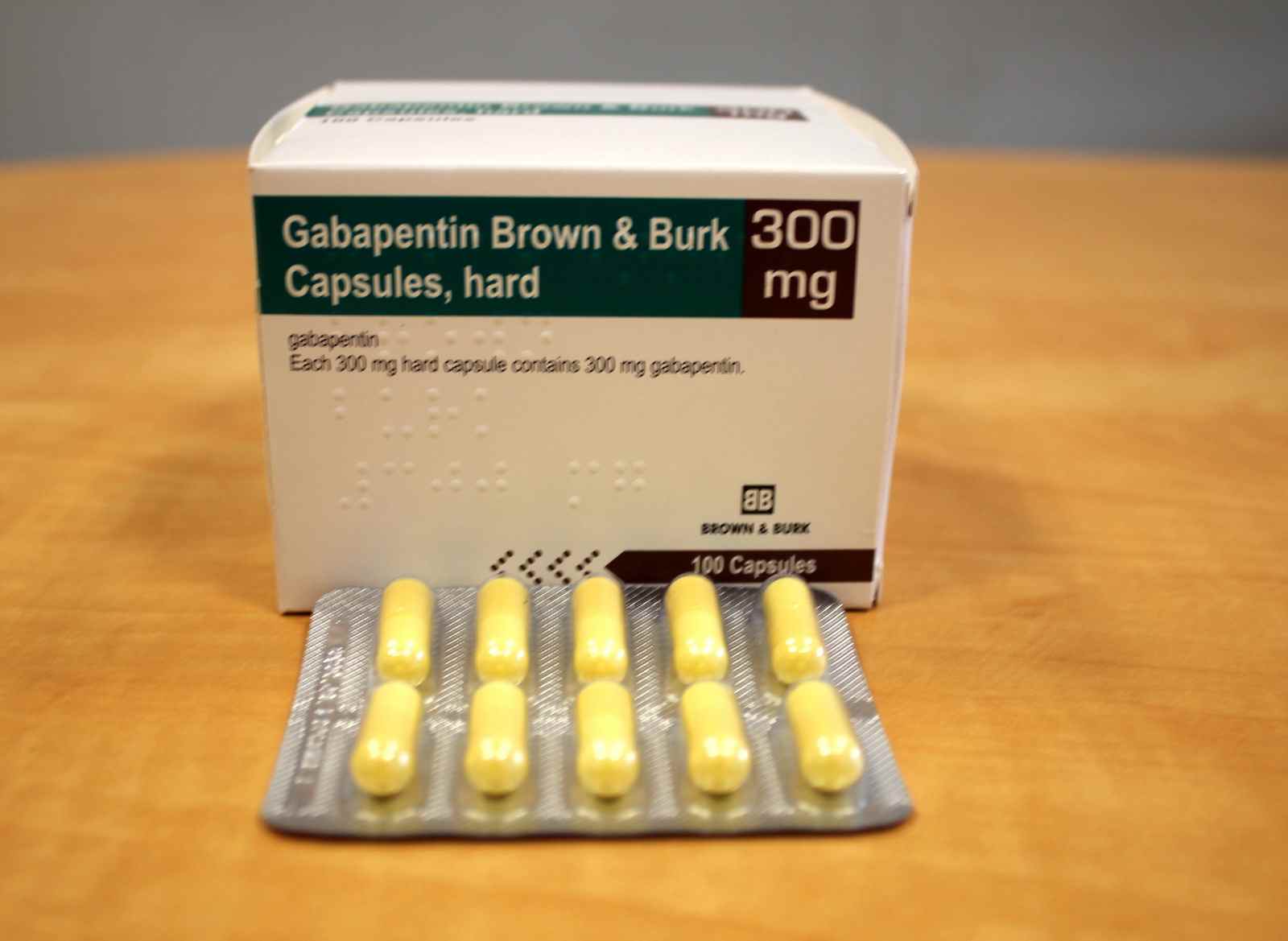 |
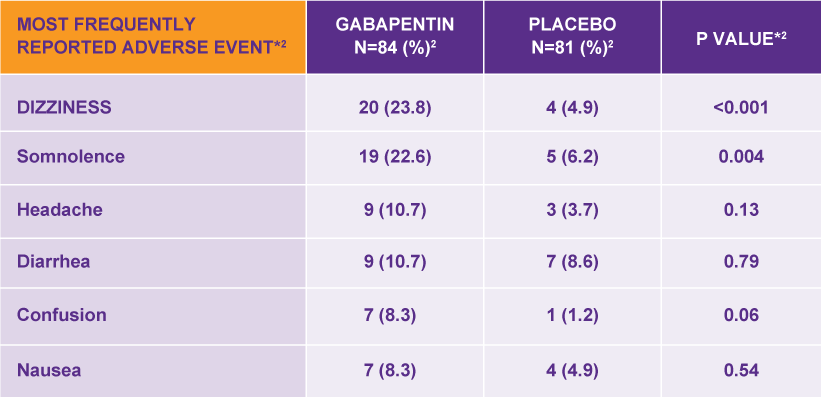
Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of Neurontin 300 mg ja 400 mg kovat kapselit sisältävät laktoosia Neurontin-kapselit sisältävät laktoosia (eräs sokeri). Jos lääkäri on kertonut, että sinulla on jokin sokeri-intoleranssi, keskustele lääkärisi kanssa ennen tämän lääkevalmisteen ottamista. Neurontin 300 mg ja 400 mg kovat kapselit sisältävät natriumia Nous ne pouvons répondre qu'aux questions générales sur les médicaments ou les stratégies de santé. Sur un plan général, la dose antalgique du Neurontin va de 900 à 3600mg chez l'adulte, en l'absence d'insuffisance rénale ou de maladie grave du foie. Elle doit être atteinte progressivement. Formulation/presentation: Neurontin ® (Gabapentin) capsule is available in 100 mg × 50’s and 300 mg × 100’s. The tablet is available in 600 mg × 100’s. Indications: Adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children >3 yrs. Neurontin is used in adults to treat neuropathic pain (nerve pain) caused by herpes virus or shingles (herpes zoster). Neurontin is also used to treat seizures in adults and children who are at least 3 years old. Use only the brand and form of gabapentin your doctor has prescribed. NEURONTIN (gabapentin) Page 9 of 36: Discontinuation of Treatment with NEURONTIN: As with other anticonvulsant agents, abrupt withdrawal is not recommended because of the possibility of increased seizure frequency. There have been post-marketing reports of adverse events such as anxiety, insomnia, nausea, Who distributes NEURONTIN. Viatris Pty Ltd Level 1, 30 The Bond 30-34 Hickson Road Millers Point NSW 2000 www.viatris.com.au Phone: 1800 274 276. This leaflet was prepared in January 2025. NEURONTIN ® is a Viatris company trade mark. Neurontin_cmi\Jan25/00. Published by MIMS February 2025 최초품목허가일: 2005.10.06 (100 mg, 400 mg), 2005.11.23 (300 mg) 최종변경허가일: 2022.04.05 NEURONTIN is used in combination with other medications to treat epilepsy in adults. How does NEURONTIN work? NEURONTIN belongs to the family of medicines called antiepileptic drugs. GABAPENTIN CAPSULES, FOR ORAL USE: Neurontin ® Prescribing Information: Medication Guide: GABAPENTIN ORAL SOLUTION Neurontin ® Prescribing Information: Medication Guide: GABAPENTIN TABLETS, FOR ORAL USE: Neurontin ® Prescribing Information: Medication Guide: GEMFIBROZIL TABLETS, USP: Lopid ® Prescribing Information: Prescribing Information Si vous prenez en même temps GABAPENTINE VIATRIS et un antiacide contenant de l'aluminium et du magnésium, l'absorption de GABAPENTINE VIATRIS à partir de l'estomac pourrait être réduite. Il est donc recommandé de prendre GABAPENTINE VIATRIS au plus tôt deux heures après avoir pris un antiacide. NEURONTIN, TABLETS gabapentin 0071-0513-24 600 mg Bottles of 100 Tablets NEURONTIN, TABLETS gabapentin 0071-0401-24 800 mg Bottles of 100 Tablets NEURONTIN, ORAL SOLUTION gabapentin 0071-2012-23 250 mg/5 mL Glass bottles containing 470 mL Oral Solution NEURONTIN ® is indicated for: In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Darf Neurontin während einer Schwangerschaft oder in der Stillzeit eingenommen / angewendet werden? Wenn Sie schwanger sind oder es werden möchten, sollen Sie Neurontin ausschliesslich nach Rücksprache mit Ihrem Arzt bzw. Ihrer Ärztin anwenden. Gabapentin, der Wirkstoff von Neurontin, geht in die Muttermilch über. VIATRIS. Vaikuttava aine. gabapentiini. Käyttötarkoitus. Lääkettä käytetään epilepsian hoitoon joko yksinään tai muiden lääkkeiden lisänä. Lääkettä käytetään myös hermovauriokivun eli ns. perifeerisen neuropaattisen kivun hoitoon. NEURONTIN is not indicated for use in children under 18 years of age. (See . 1.1 Pediatrics; 7.1.3 Pediatrics). Hepatic Impairment: Because gabapentin is not metabolized to a significant extent in humans, no studies have been performed in patients with hepatic impairment. 4.4 Administration NEURONTIN is given orally with or without food. Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) The products on this page have been transferred to VIATRIS SPECIALTY LLC. The U.S. Physician Prescribing Information and Patient Information on this page may no longer be current. Please visit Viatris.com for the most current product information. for Consumers: NEURONTIN U.S. Medication Guide; for Healthcare professionals: neurontin ® Content: Gabapentin Indication: Adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 3 years and older. Non sospenda il trattamento con Neurontin a meno che non glielo abbia detto il medico. La sospensione del trattamento deve avvenire in modo graduale nell’arco di almeno 1 settimana. Se sospende improvvisamente il trattamento con Neurontin oppure prima che le sia stato prescritto dal medico, il rischio di attacchi epilettici aumenta.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |