Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
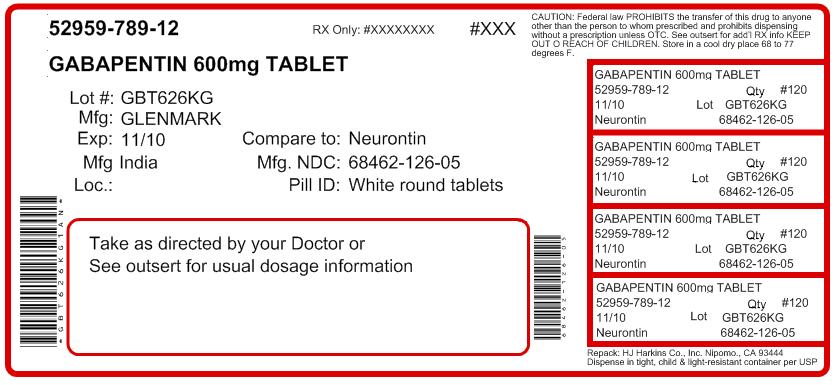
Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). Despite these limited indications, gabapentin and pregabalin are widely prescribed off-label for various other pain syndromes. Such use is growing, possibly because clinicians are searching increasingly for alternatives to opioids. Like gabapentin, it is sometimes used with opiates, with toxic or even lethal results. Similarly, when in combination with alcohol or nervous system depressants, there is the possibility of greater toxicity. Choosing gabapentin and pregabalin: These drugs are widely used off-label as an alternative to benzodiazepines for anxiety disorders. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. for an off-label use. Examples of Off-Label Medication Use for Mental Health Conditions . Amitriptyline • Insomnia • Posttraumatic stress disorder (PTSD) Clonidine • Smoking cessation • Excessive saliva caused by clozapine Gabapentin • Alcohol dependence • Social anxiety Prazosin • Post traumatic stress disorder Methods: National Ambulatory Care Medical Survey data (2011-2016) were used to identify encounters involving gabapentin (gabapentin visits) for adults (ages ≥18) (N=5,732). FDA-approved uses and off-label psychiatric use indications were identified with ICD-9-CM and ICD-10-CM diagnosis codes. CNS-D drugs examined were opioids, benzodiazepines Our analysis compared study protocols for off-label use of gabapentin and the manufacturers' internal research reports with the published reports of study findings. The results of this analysis OBJECTIVES: (1) Describe the relevance of off-label use of gabapentin to man-aged care pharmacy; (2) summarize recent FDA warnings and media reports related to off-label gabapentin use; (3) review medical information pertaining to the off-label use of gabapentin; (4) outline alternatives to off-label use of Because gabapentin is FDA approved for the treatment of postherpetic neuralgia in adults and as an adjunct therapy for the treatment of partial-onset seizures, the use of this medication for anything other than these indications is considered off-label. Perhaps one of the more promising off-label uses for Gabapentin is for the treatment of anxiety disorders. There is mounting evidence that Gabapentin may be an effective intervention for various types of anxiety including: generalized anxiety disorder, social anxiety disorder, and panic disorder. This study examined off-label use of gabapentin for psychiatric indications and its concomitant use with CNS-D prescription drugs in a nationally representative sample of ambulatory care office visits. Less than 1% of outpatient gabapentin use was for FDA-approved indications. For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralgia. For pregabalin, FDA-approved indications related to pain are limited to postherpetic neuralgia, neuropathic pain associated with diabetic neuropathy or spinal cord injury, and fibromyalgia. Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. While Gabapentin is FDA-approved for partial seizures and postherpetic neuralgia, its off-label uses are more extensive, especially in psychiatry. Gabapentin for anxiety disorders is notable, with doses between 900 and 3,600 milligrams per day showing effectiveness in reducing symptoms. Gabapentin’s off-label uses—meaning prescribing gabapentin for a problem not on the FDA approval list for this medication—that physicians are finding include: Alcohol withdrawal; Anxiety; Cocaine withdrawal; Fibromyalgia; Headaches; Hiccups; Hot flashes; Hyperhidrosis; Insomnia; Migraines; Mood disorders; Pain syndromes; Peripheral Gabapentin is frequently prescribed off-label for various pain syndromes, including chronic pain and neuropathic pain, despite limited FDA-approved indications. This trend is partly driven by the search for alternatives to opioid medications. Vedula SS, Bero L, Scherer RW, et al. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361(20):1963-1971. Goodman CW, Brett AS. A clinical In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 We selected gabapentin (Neurontin), a medication reported to be widely used off label, as a specific example to explore specialist physicians' experiences with off-label prescribing. The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |