Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
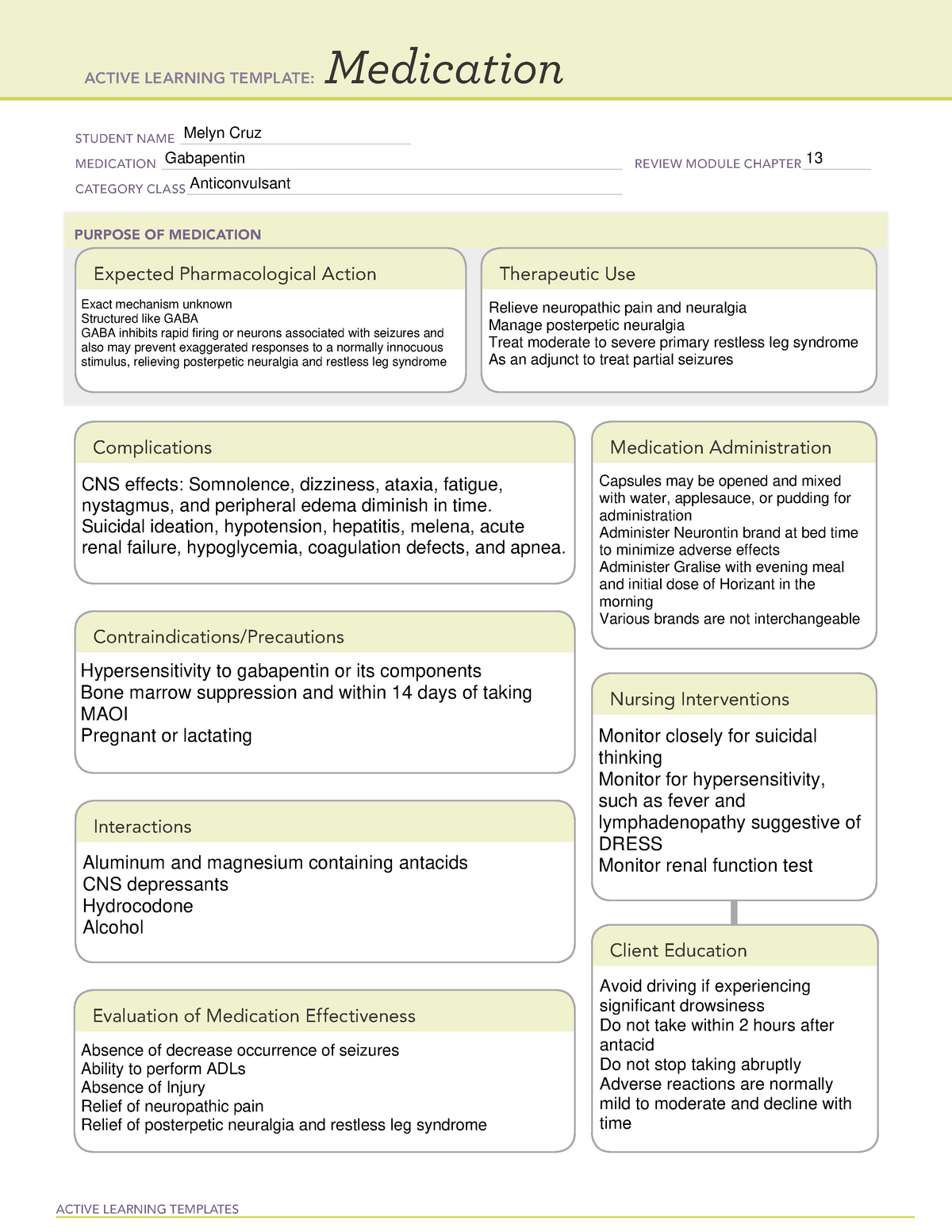 |  |
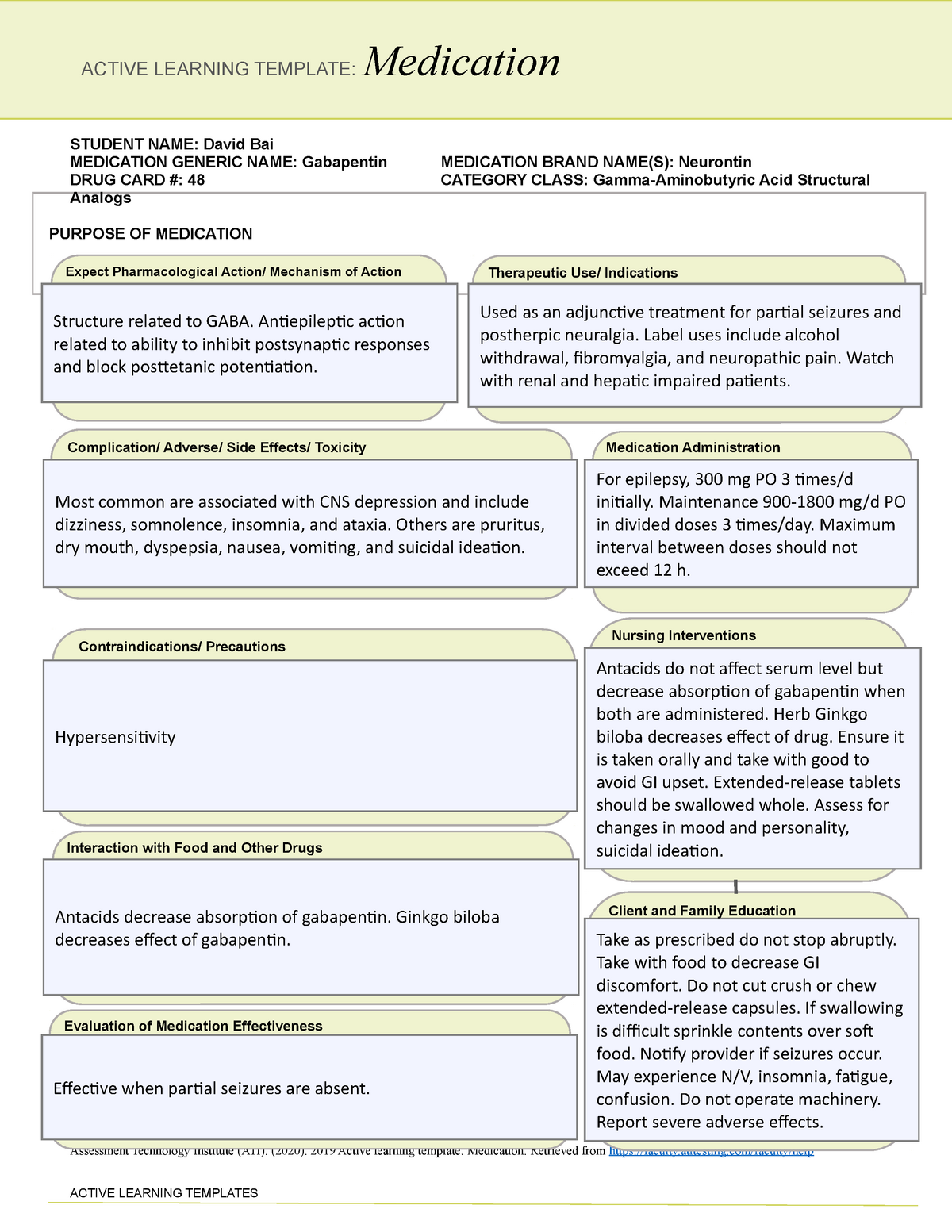
Gabapentin package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. solutions. The chemical structures for gabapentin, GABA, and pregabalin are shown below: NH 2 OH O NH 2 OH O NH 2 OH O Gabapentin GABA Pregabalin. Pharmacology: The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin Gabapentin in the management of restless legs syndrome (RLS) has been evaluated in small controlled trials, demonstrating benefits compared with placebo. Gabapentin enacarbil is FDA-approved for the treatment of RLS Garcia-Borreguero 2002, Saletu 2010. The . Social anxiety disorder, adjunct to antidepressants or monotherapy (alternative agent)c Instruct patient to take medication exactly as directed. Patients on 3 times daily dosing should not exceed 12 hr between doses. Take missed doses as soon as possible; if less than 2 hr until next dose, take dose immediately and take next dose 1-2 hr later, then resume regular dosing schedule. PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Gabapentin is also active in models that detect anxiolytic activity. Gabapentin | C9H17NO2 | CID 3446 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Gabapentin is in a class of medications called anticonvulsants. Gabapentin treats seizures by decreasing abnormal excitement in the brain. Gabapentin relieves the pain of PHN by changing the way the body senses pain. Although it has been known for some time that gabapentin must bind to the α 2 δ-1 protein in order to act pharmacologically (see Pharmacodynamics), the three-dimensional structure of the α 2 δ-1 protein with gabapentin bound (or alternatively, the native amino acid, L-Isoleucine bound) has only recently been obtained by cryo-electron Indicated for prevention of partial seizures, for restless leg syndrome, in neuropathic pain, for post-heppetic neuralgia, as a co-analgesic, and in alcohol withdrawal. Adverse effects are few, but do include Steven-Johnson syndrome, but more commonly just ataxia and somnolence. While gabapentin's mechanism of action is generally understood, it appears to be a pharmacologic option for treating issues involving the gamma-aminobutyric acid (GABA) receptor system. Gabapentin is a relatively safe, readily available, and effective drug for alcohol-use disorder treatment, specifically for the abstinence maintenance phase. Gabapentin capsules are given orally with or without food. Gabapentin capsules should be swallowed whole with plenty of water. If gabapentin dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Gabapentin is an anticonvulsant medication used in the management of peripheral neuropathic pains, postherpetic neuralgia, and partial-onset seizures. Gabapentinoids, also known as α 2 δ ligands, are a class of drugs that are chemically derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) (i.e., GABA analogues) which bind selectively to the α 2 δ protein that was first described as an auxiliary subunit of voltage-gated calcium channels (VGCCs).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |