Gallery
Photos from events, contest for the best costume, videos from master classes.
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/7RXMEOM35FFEFBAMLZK7HWIWUM.jpg) |  |
 |  |
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc():focal(749x0:751x2)/cardi-b-no-makeup-040623-89ae061ffe354c699f4eb9047634339f.jpg) |  |
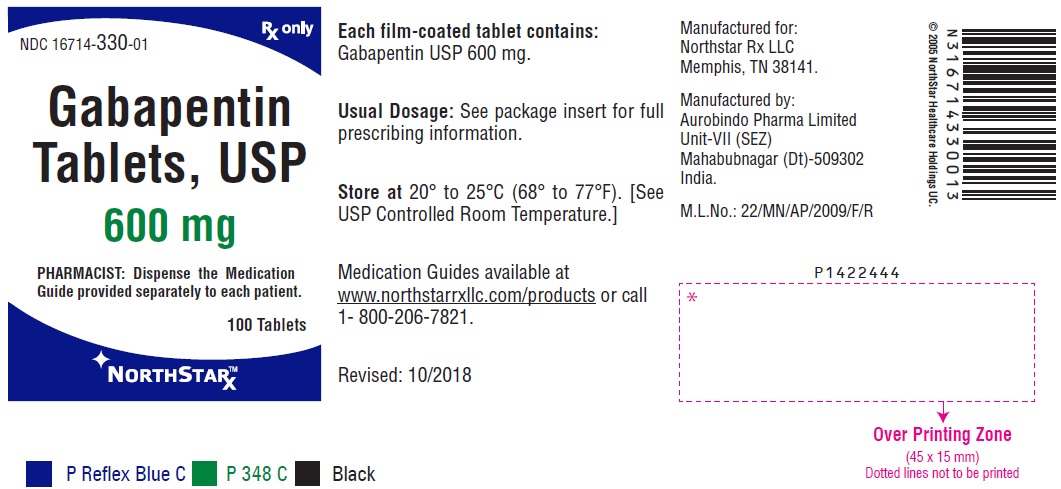
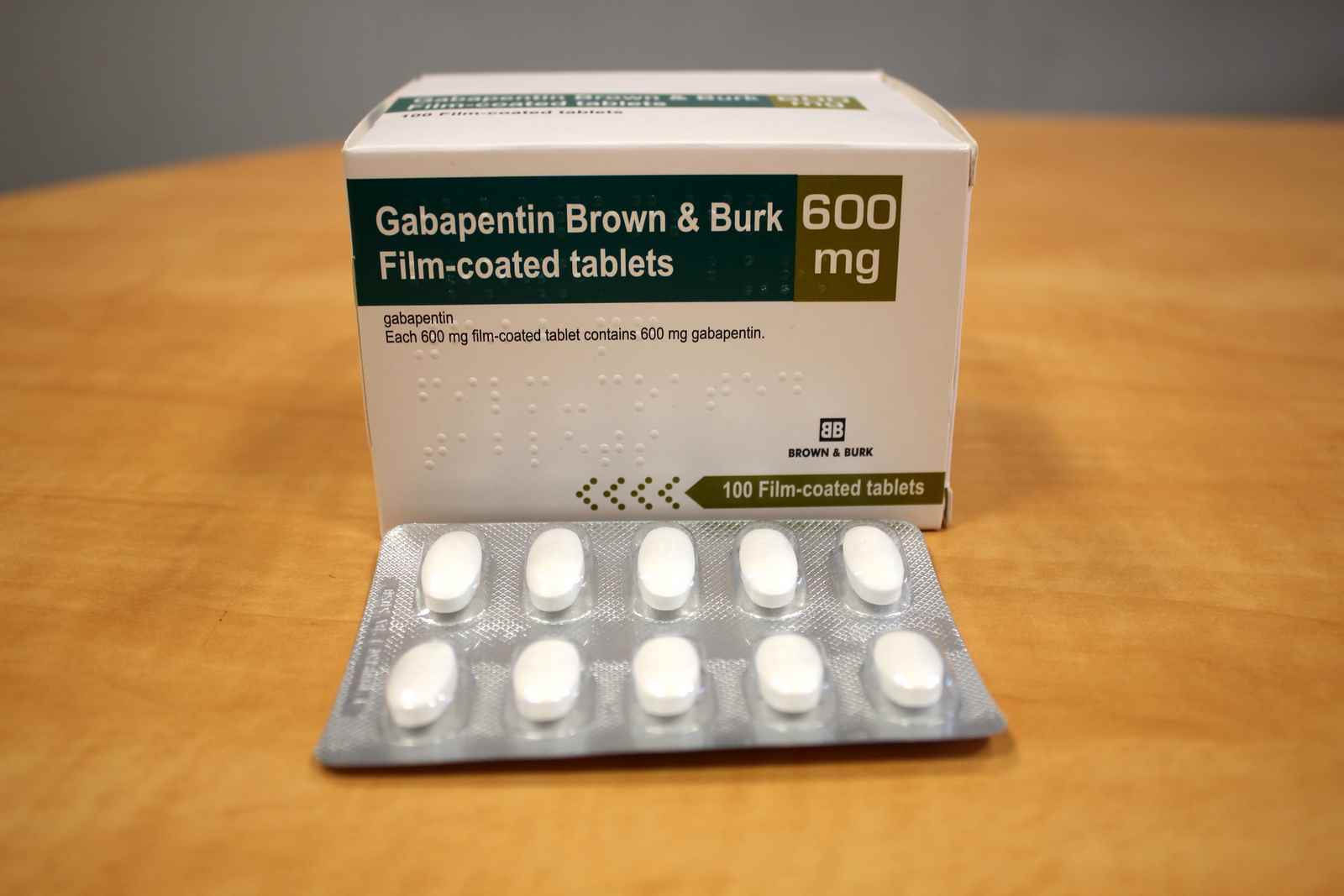
FDA Announces Voluntarily Recall for Batch of Gabapentin 300 MG Capsules Epilepsy News From: Tuesday, November 25, 2014 The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. On December 19, 2019 FDA is warning that serious breathing difficulties may occur in patients using gabapentin (brand names Neurontin, Gralise, Horizant) or pregabalin (brand names Lyrica, Aurobindo Pharma USA, Inc has issued a voluntary recall of one lot of gabapentin capsules, USP 300 mg, 100-count bottles to the consumer level after finding that some capsules were empty. Mandated? Product mixup: one foreign tablet found in product. The last Recall Enforcement Report for Gabapentin with NDC 70010-227 was initiated on 07-31-2024 as a Class II recall and it is currently ongoing. Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., originally initiated on 07-31-2024 for the product Gabapentin Tablets, USP, 600 mg, 500-count bottles, Rx only, Manufactured by: Granules India Limited Hyderabad-500 081, India, Manufactured for: Granules Pharmaceuticals Inc., Chantilly, VA NDC 70010 The last Recall Enforcement Report for Gabapentin with NDC 70010-228 was initiated on 07-31-2024 as a Class II recall due to presence of foreign tablets; 3 fused tablets of metformin er 500 mg were found in bottle of gabapentin tablets The latest recall number for this product is D-0634-2024 and the recall is currently ongoing . Get an alert when a recall is issued. Do not use • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. Drug Recall Enforcement Report Class III voluntary initiated by Sciegen Pharmaceuticals Inc, originally initiated on 02-17-2023 for the product Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors encourages pharmacies to immediately review their quality assurance and recall policies and procedures to determine if any corrective action is required. GRANULES PHARMACEUTICALS INC. is recalling Gabapentin Tablets USP, 600mg. This recall is due to a potential mix-up of Metformin ER Tablets in a bottle of Gabapentin Tablets, USP 600mg. Using data from a large national survey, Johansen found that 4.7% of U.S. adults were prescribed a gabapentinoid in 2021, up from 4% in 2015 – a statistically significant increase of 17.5% in six years. The growth was primarily driven by gabapentin, as there was little change in pregabalin’s use. Gabapentin (Neurontin) and pregabalin (Lyrica) are both gabapentinoids—psychotropic medications that cross the blood-brain barrier and mimic the inhibitory neurotransmitter Gamma-aminobutyric acid (GABA). Gabapentin was first approved by the Food and Drug Administration (FDA) in 1993 as an adjunctive treatment for partial seizures. In 2002 By 2019, gabapentin was so common among overdose deaths in Kentucky that the state declared it a controlled substance. Gabapentin was detected in a quarter of all overdose deaths in Louisville, the state’s largest city, in 2018. At the time, the FDA had not yet established gabapentin’s breathing-related risks. The Harvard Drug Group, LLC d/b/a Major Pharmaceuticals and Rugby Laboratories has initiated a recall for Gabapentin Tablets, 600 mg. This recall has been initiated due to a product complaint where one incorrect tablet, identified as Atorvastatin Calcium Tablets, 40 mg, was found in blister packaging for Gabapentin Tablets, USP, 600 mg. I'm going to taper off the gabapentin and hope my cognitive functioning improves. I knew the detached, spacey, and scatterbrained way I've been feeling was probably the gabapentin, but it's the severe lack of recall, often in mid-sentence when talking that scares me. Thanks for sharing everyone. Gabapentin is being recalled due to a potential issue with empty capsules within certain batches. This isn’t a widespread recall of all gabapentin products, but rather a specific batch of 300 mg capsules manufactured by Aurobindo Pharma USA. The Harvard Drug Group is pulling 3984 cartons of gabapentin tablets after a foreign tablet was found in a carton, according to the May 17, 2023, US Food and Drug Administration (FDA) Enforcement Report. Taking gabapentin or pregabalin with opioids, anxiety meds or antidepressants, or if you have lung issues or are elderly, can lead to serious breathing problems. FDA provides a searchable list of recalled products. Drug recalls are actions taken by a firm to remove a product from the market and may be conducted on a firm's own initiative, by FDA request
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/7RXMEOM35FFEFBAMLZK7HWIWUM.jpg) |  |
 |  |
 |  |
 |  |
 |  |
:max_bytes(150000):strip_icc():focal(749x0:751x2)/cardi-b-no-makeup-040623-89ae061ffe354c699f4eb9047634339f.jpg) |  |