Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
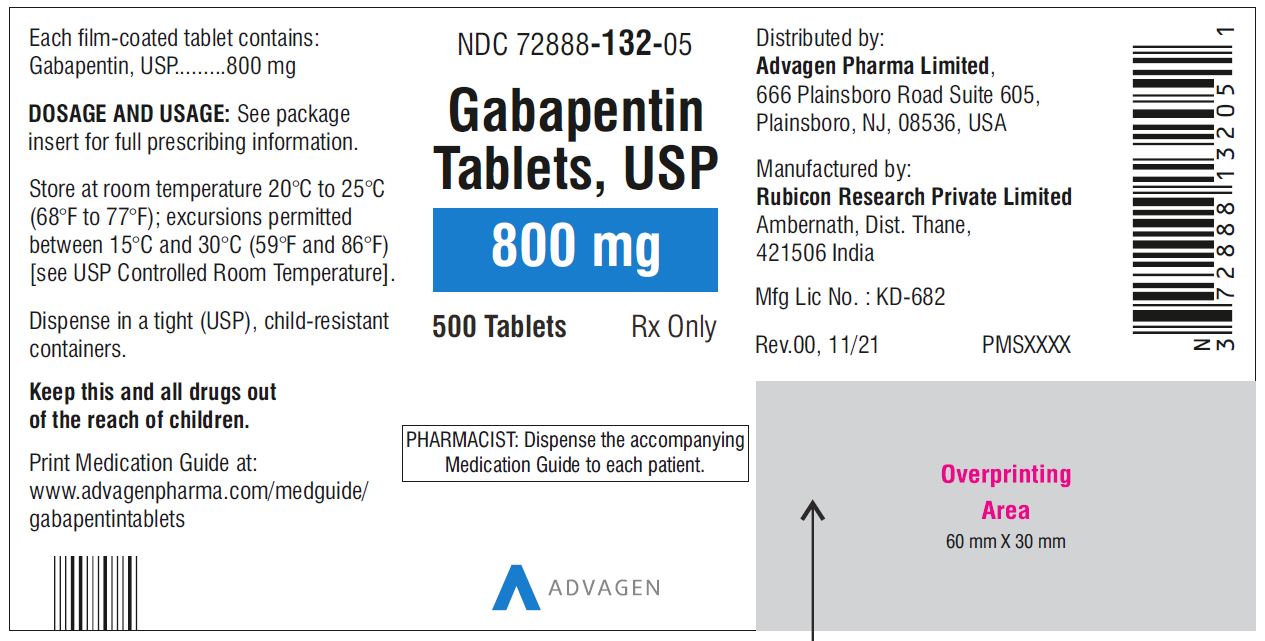 |  |
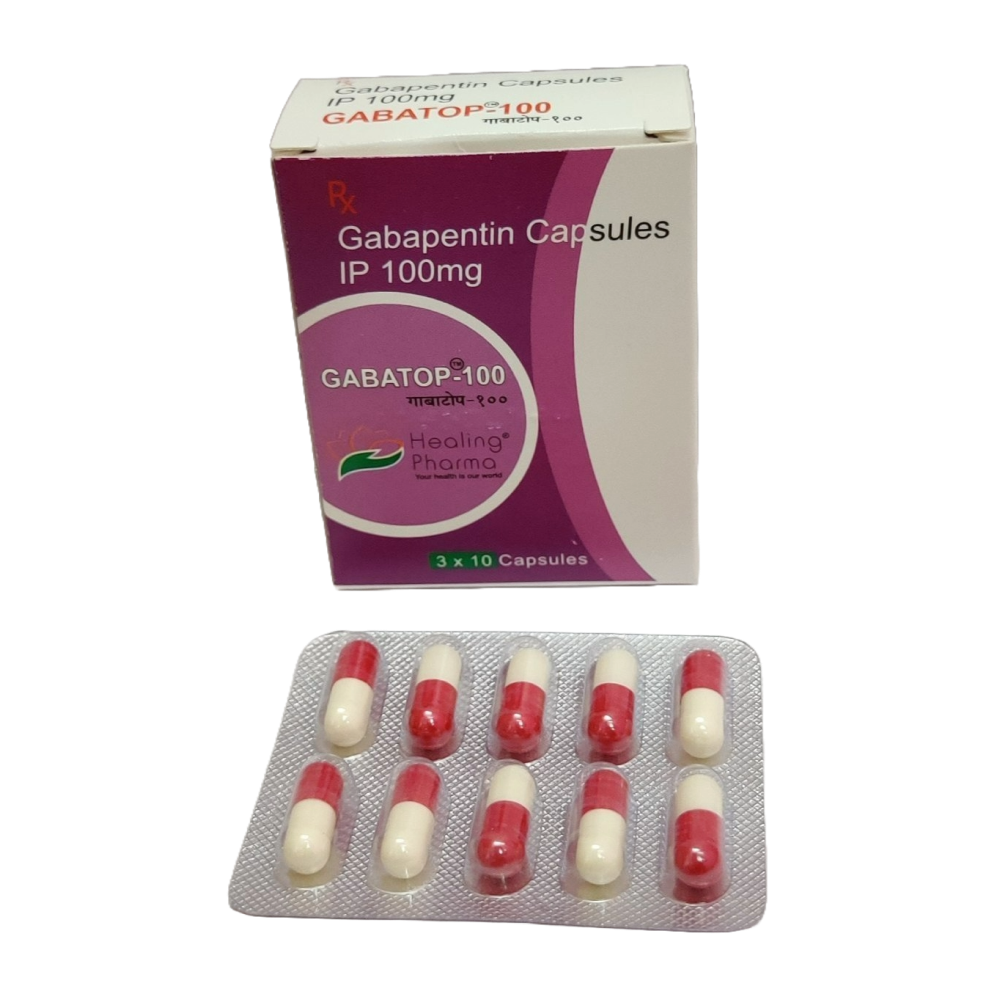
single substance pharmaceutical exposures (i.e., the number of human exposure cases that identified only one substance), gabapentin was identified as a single substance in 6,955 cases in 2022. With respect to medical outcomes associated with gabapentin calls to poison control centers in 2022, gabapentin was associated with 6 deaths, 164 Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Gabapentin 300 mg is not a controlled substance under the Controlled Substances Act (CSA). Images for SG 180 Pill with imprint 216 is White, Capsule/Oblong and has been identified as Gabapentin 100 mg. It is supplied by Ascend Laboratories, LLC. Brand names of gabapentin include Horizant®, Gralise® and Neurontin®. What is gabapentin approved for? Gabapentin is used to: Prevent and control partial seizures. Gabapentin can be used in adults and children age 3 and older who have partial seizures. Relieve nerve pain following shingles in adults. Taking gabapentin can make you sleepy. According to studies, about 20% of people taking gabapentin experience drowsiness or fatigue. It may be even more likely, affecting 20% to 30% of people, with Horizant. In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a Pill with imprint 215 is Yellow, Capsule/Oblong and has been identified as Gabapentin 300 mg. It is supplied by Ascend Laboratories, LLC. Gabapentin is used in the treatment of Back Pain; Postherpetic Neuralgia; Epilepsy; Chronic Pain; Seizures and belongs to the drug class gamma-aminobutyric acid analogs. Risk cannot be ruled out during pregnancy. Use our Pill Identifier tool to instantly match by imprint, shape, color, drug name, or NDC code. Access over 11,500 drug images, updated daily. Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. 4. Do prescribers need a DEA number to prescribe Gabapentin? • No, a DEA number is not required to prescribe Gabapentin. 5. As a dispenser, how do report gabapentin if I don’t have a DEA number? • There are three identifiers available for dispensers to report gabapentin: i. Drug Enforcement Administration (DEA) number ii. Find all the important details about this NDC Package code, including the 11-Digit NDC Billing number, billing units, wholesale price, RxNorm crosswalk, active ingredients, pharmacologic clasess, etc. Gabapentin is used with other medications to prevent and control seizures. Several small studies indicate that gabapentin can reduce the itching associated with kidney failure. But the data for its effectiveness against low back pain or a number of psychiatric disorders Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Drug Identifier results for "Gabapentin". Search by imprint, shape, color or drug name. 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and The total number of patients treated with gabapentin in controlled clinical trials in patients with postherpetic neuralgia was 336, of which 102 (30%) were 65 to 74 years of age, and 168 (50%) were 75 years of age and older. List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Gabapentin. Side 1: 215. Side 2: N/A. View Free Coupon. Famotidine. Side 1: L194. A pill’s imprint code can be made up of any single letter or number, or any combination of letters, numbers 36. Is Gabapentin a Controlled Substance in Tennessee and does it require a DEA to prescribe? Gabapentin is a Schedule V Controlled Substance in Tennessee and therefore should be treated just like any other Schedule V Controlled Substance. 37. I suspect my healthcare practitioner is engaged in TennCare fraud, waste, or abuse. What do I do? The total number of patients treated with gabapentin in controlled clinical trials in patients with postherpetic neuralgia was 336, of which 102 (30%) were 65 to 74 years of age, and 168 (50%) were 75 years of age and older.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |