Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 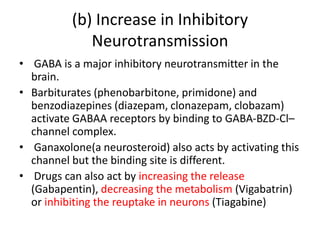 |
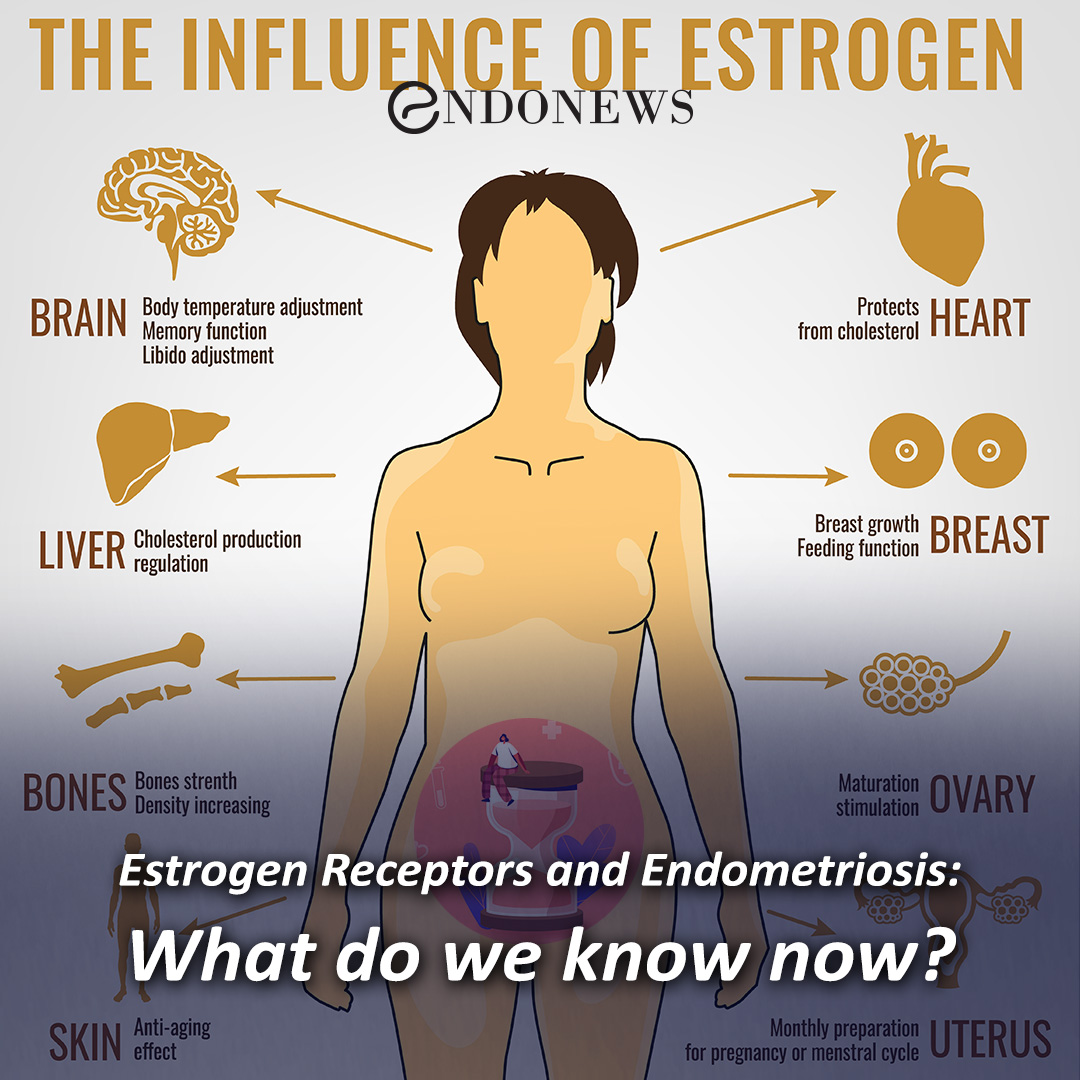
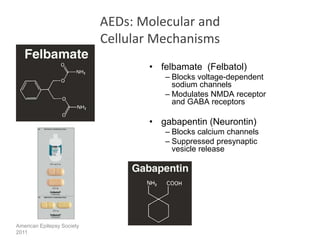
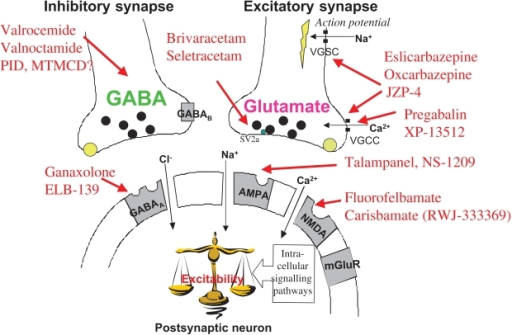
This study suggested that the antiepileptic GABA analogue gabapentin (Neurontin) is an agonist at GABA(B) receptors expressing the GABA(B1a) but not the GABA(B1b) receptor subunit. Although it is rapidly absorbed, readily crosses the blood–brain barrier and is orally active in several animal models of epilepsy, gabapentin neither binds to GABA A or GABA B receptors nor is it metabolized to GABA (Goa and Sorkin, 1993; Kammerer et al, 2011; Taylor et al, 1992). Several mechanisms of gabapentin have been proposed after neuropathy including an inhibition of NMDA receptors, inhibition of sodium currents and reducing β4a subunit mediated VGCC trafficking (Hara and Sata 2007; Mich and Horne 2008; Yang et al. 2009). Neither GBP nor PGB has any appreciable effect on the GABA A receptor complex [11, 12••]. A series of recent research reports has, however, addressed the possibility that GBP may exert its effects, at least in part, by activation of presynaptic GABA B receptors. In addition to a structural similarity to baclofen, GBP was initially reported Gabapentin (Neurontin®) is a second-generation antiepileptic drug widely used for treatment of neuropathic pain. It is also used to treat anxiety, insomnia, bipolar disorder, and restless leg syndrome. Although first introduced as an adjunct therapy for epilepsy, gabapentin became a blockbuster drug for the management of chronic pain from many nerve conditions [8]. Side effects are usually Gabapentin is a structural analog of the inhibitory neurotransmitter γ-aminobutyric acid (GABA). Its anticonvulsant, analgesic and anxiolytic properties suggest that it increases GABAergic inhibition; however, the molecular basis for these effects is unknown as gabapentin does not directly modify GABA type A (GABA A) receptor function, nor does it modify synaptic inhibition. Keywords: Gabapentin, pregabalin, pain management, adverse effects, pharmacology. Introduction. The gabapentinoid drugs gabapentin and pregabalin are antiepileptic drugs that are considered as first-line treatments for the management of neuropathic pain. 1 Pregabalin is also approved for generalised anxiety disorders in the United Kingdom. The It is concluded that gabapentin is not an agonist at GABAB receptors that are functional in baclofeninduced antiallodynia in the postoperative pain model in vivo and in GIRK channel activation in ventrolateral PAG neurons in vitro. Evidence linking gabapentin to the NMDA receptor follows research demonstrating the reversal of the antihyperalgesic effect of gabapentin by d-serine, an agonist at the NMDA-glycine binding site [33, 34, 36, 37]. However, receptor binding studies have failed to demonstrate a direct binding site for gabapentin at the NMDA receptor . Gabapentin, a novel anticonvulsant and analgesic, is a Á-aminobutyric acid (GABA) analogue but was shown initially to have little affinity at GABA A or GABA B receptors. It was recently reported to be a selective agonist at GABA B receptors Similarly in cultured tissue slices, gabapentin does not consistently inhibit transmitter release (Fink et al. 2000; Fehrenbacher et al. 2003; Brown and Randall 2005; Quintero et al. 2011). These findings are perhaps unsurprising given they were performed in normal tissue and the analgesic effects of gabapentinoids are mainly revealed by Gabapentin and pregabalin are structurally similar to gamma-aminobutyric acid (GABA), although they do not bind to GABA receptors. They bind to the α-2-delta subunit of voltage-dependent calcium channels (aka alpha-2-delta calcium channel ligands). It is concluded that gabapentin is not an agonist at GABA (B) receptors that are functional in baclofen-induced antiallodynia in the postoperative pain model in vivo and in GIRK channel In the present study, we examined whether gabapentin is an agonist at native GABA(B) receptors using a rat model of postoperative pain in vivo and periaqueductal gray (PAG) slices in vitro; PAG contains GABA(B) receptors, and their activation results in antinociception. To understand how gabapentin might influence dopamine levels, we first need to explore its primary mechanisms of action. Contrary to its name, gabapentin does not directly interact with GABA receptors or influence GABA levels in the brain. Instead, its primary mode of action involves modulating voltage-gated calcium channels. Gabapentin has no activity at GABAA or GABAB receptors of GABA uptake carriers of brain. Gabapentin interacts with a high-affinity binding site in brain membranes, which has recently been identified as an auxiliary subunit of voltage-sensitive Ca2+ channels. GABA exerts its inhibitory effect through two types of specific receptors, GABA_A (ionotropic) and GABA_B (metabotropic), which show different pharmacological, structural, and molecular differences. GABA receptors are the most common in the nervous system. Research has shown that gabapentin exerts a modulating effect at neuronal receptor sites, inhib- iting the release of the neurotransmitters dopamine (5), serotonin and norepinephrine (6) and resulting in in- creased GABA concentrations in various locations throughout the brain (7). Gabapentin was formed by the addition of a cyclohexyl group to GABA, which allowed this form of GABA to cross the blood–brain barrier. Despite its structural similarity to GABA, gabapentin does interact with GABA receptors in the CNS. Its mechanism of action is unknown, but may involve enhanced neuronal GABA synthesis. Gabapentin robustly increases cell-surface expression of δGABA A receptors and increases a tonic inhibitory conductance in neurons. This enhanced δGABA A receptor function contributes to the ataxic and anxiolytic but not antinociceptive properties of gabapentin.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |