Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |
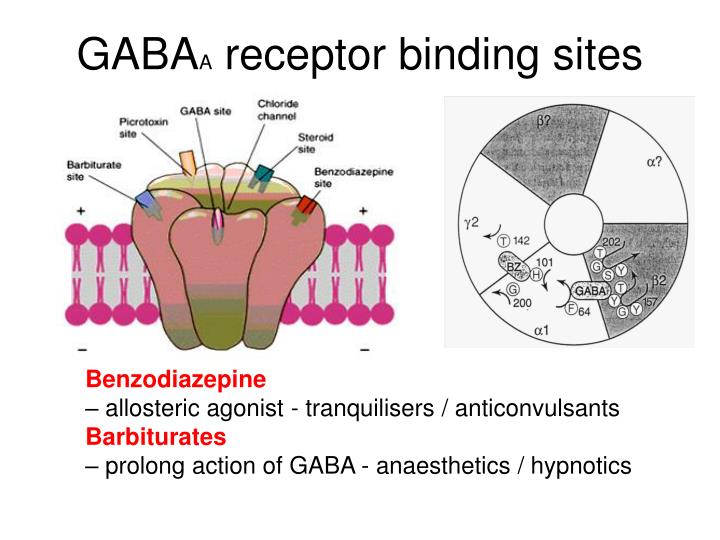
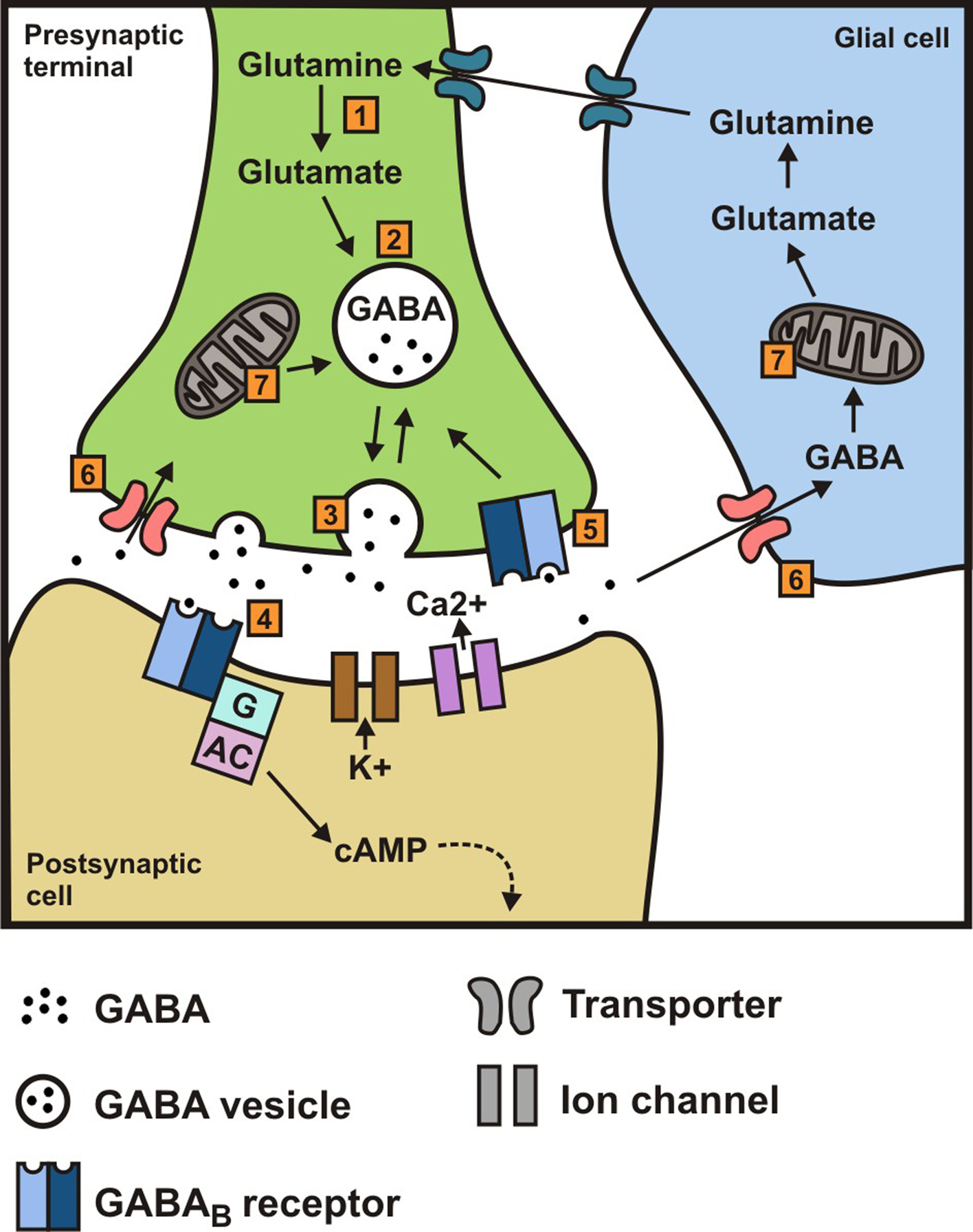
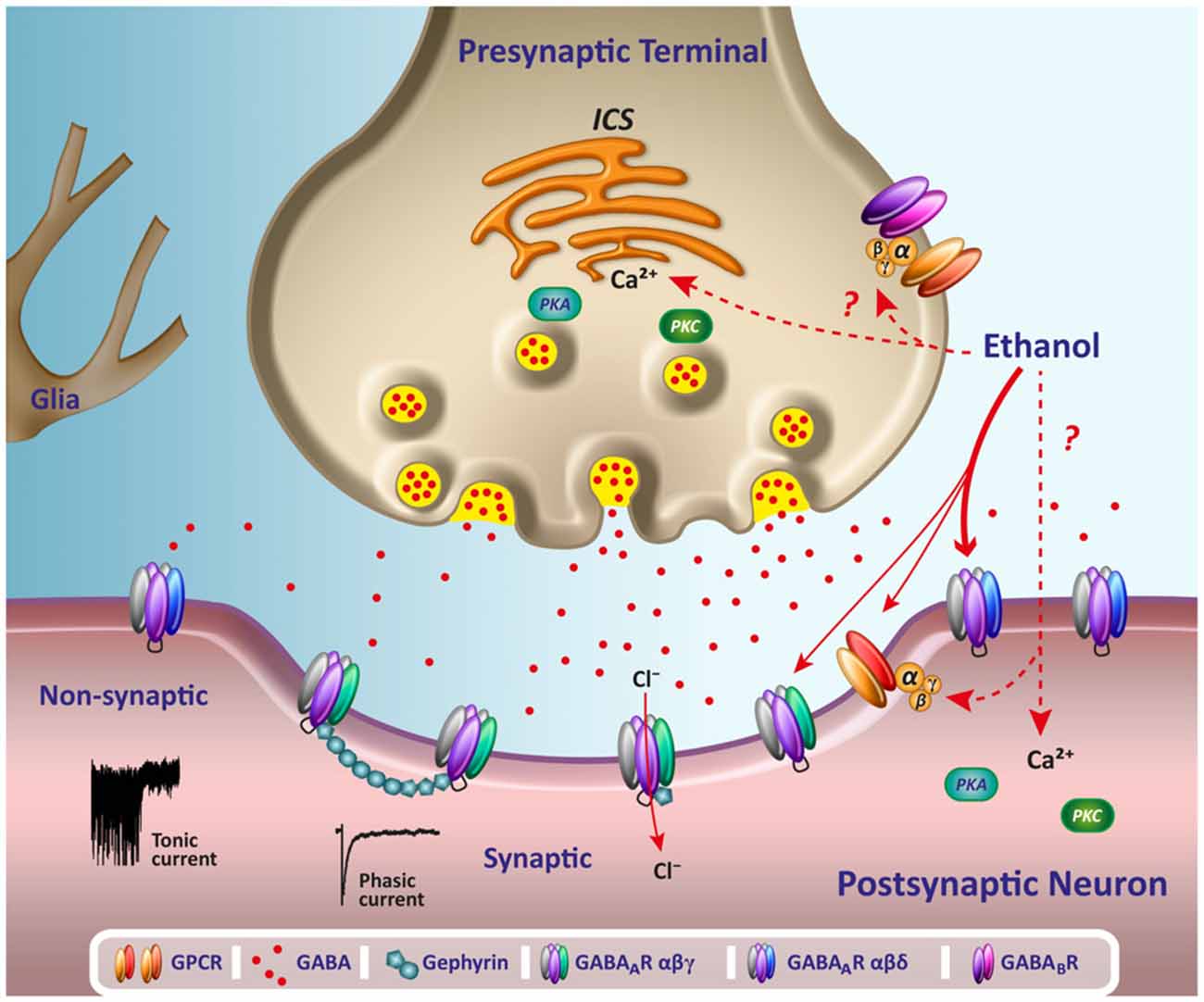
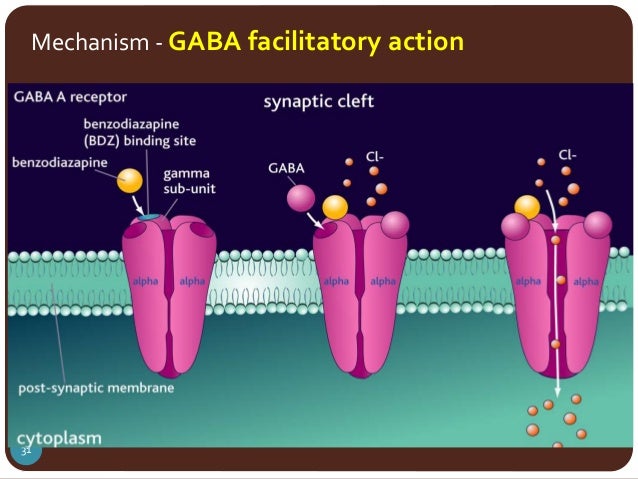
However, receptor binding studies have failed to demonstrate a direct binding site for gabapentin at the NMDA receptor . The α 2 δ subunit of the voltage-dependent calcium channel is a binding site for gabapentin and the S-isomer of pregabolin (S-(+)-3-isobutylgaba) [4, 33, 37, 41]. Gabapentin is a structural analog of GABA, which readily crosses the blood-brain barrier when given systemically . Gabapentin, however, does not bind to GABA receptors . Although gabapentin may influence the synthesis and release of GABA , it does not affect the uptake and metabolism of endogenous GABA . Specifically, gabapentin binds to the alpha-2-delta subunit of these channels. VGCCs play a crucial role in the release of neurotransmitters by regulating calcium influx into neurons. By binding to the alpha-2-delta subunit, gabapentin reduces the release of excitatory neurotransmitters such as glutamate, norepinephrine, and substance P. Although gabapentin is a GABA analogue, it does not bind to and modulate the GABA receptors nor does it affect GABA transport or metabolism. Gabapentin is a gabapentinoid, which acts as an inhibitor of the α2δ subunit-containing voltage-dependent calcium channels (VDCCs) that are linked to neurotransmitter release. Gabapentin (Neurontin) Primer Gabapentin (Trade name: Neurontin) is an anticonvulsant. It is commonly also used off-label for anxiety disorders, restless leg syndrome, and in alcohol use disorder. It is structurally similar to GABA but does not directly bind to GABA receptors. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. The α 1 subunit allows entry of calcium and the extracellular α 2 δ is bound to the γ The gabapentinoid drugs gabapentin and pregabalin are key front‐line therapies for various neuropathies of peripheral and central origin. Originally designed as analogs of GABA, the gabapentinoids bind to the α 2 δ‐1 and α 2 δ‐2 auxiliary subunits However, receptor binding studies have failed to demonstrate a direct binding site for gabapentin at the NMDA receptor . The α 2 δ subunit of the voltage-dependent calcium channel is a binding site for gabapentin and the S-isomer of pregabolin (S-(+)-3-isobutylgaba) [4, 33, 37, 41]. Second, the data of Chen et al. 7 do not address the fundamental question of how binding of gabapentin to Ca v α 2 δ-1 ultimately reduces the cell surface expression of Ca v 1.2 or, more Although it is rapidly absorbed, readily crosses the blood–brain barrier and is orally active in several animal models of epilepsy, gabapentin neither binds to GABA A or GABA B receptors nor is it metabolized to GABA (Goa and Sorkin, 1993; Kammerer et al, 2011; Taylor et al, 1992). Research regarding gabapentin's effects on GABA and glutamate 4. NMDA receptors The answer is 3. Gabapentin and pregabalin are structurally similar to gamma-aminobutyric acid (GABA), although they do not bind to GABA receptors. They bind to the α-2-delta subunit of voltage-dependent calcium channels (aka alpha-2-delta calcium channel ligands). Gabapentin, on the other hand, does not directly interact with GABA receptors. Instead, it binds to a specific subunit of voltage-gated calcium channels, known as the alpha-2-delta subunit. By binding to this subunit, Gabapentin reduces the release of excitatory neurotransmitters, such as glutamate, thereby decreasing neuronal excitability. While gabapentin was designed as a GABA analogue, its GABA-like properties are not as straightforward as initially believed. The drug does not directly bind to GABA receptors or increase GABA levels in the brain. However, it may indirectly enhance GABAergic neurotransmission through its effects on calcium channels and other neuronal processes. Introduction. The anticonvulsant gabapentin was first reported as providing pain relief 20 years ago (Mellick et al. 1995).The discovery that anticonvulsants could be used as analgesics started a scientific journey leading to the inclusion of gabapentinoids as key frontline therapy for various neuropathies. Gabapentin's binding site is located on the α 2 δ subunit of voltage gated calcium channels. 6 In vitro, gabapentin can inhibit neuronal calcium currents by 35% and decrease neuronal tachykinin-mediated activity. 7,8 It has been suggested that mitigation of hypothalamic tachykinin neurotransmitter activity via a decrease in neuronal calcium Gabapentin has no activity at GABAA or GABAB receptors of GABA uptake carriers of brain. Gabapentin interacts with a high-affinity binding site in brain membranes, which has recently been identified as an auxiliary subunit of voltage-sensitive Ca2+ channels. However, the functional correlate of gabapentin binding is unclear and remains under study. Despite extensive research the mechanism of action of gabapentin remains unclear. In vivo behavioral studies have suggested the possible involvement of the glycine co-agonist site of the NMDA receptor complex in the anticonvulsant action of gabapentin; intracerebroventricular administration of D-serine (a glycine site agonist) reversed the protection afforded by gabapentin against chemically Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Instead, it exhibits several distinct pharmacological activities, including: (1) binding to the alpha-2-delta protein subunit of voltage-gated calcium The gabapentinoid drugs do not bind significantly to other known drug receptors and so the α 2 δ VGCC subunit has been called the gabapentin receptor. [ 15 ] [ 4 ] Recently, the same α 2 δ-1 protein has been found closely associated not with VGCCs but with other proteins such as presynaptic NMDA-type glutamate receptors , cell adhesion have shown that gabapentin binds to auxiliary α2-δ subunits of voltage-gated Ca. 2+ channels on neurons, thereby resulting in a decrease in neuronal excitability. At clinically therapeutic doses (900– 3600 mg/day), gabapentin does not bind to GABA. A. or GABA. B. receptors, nor does it bind to benzodiazepine sites.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |