Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
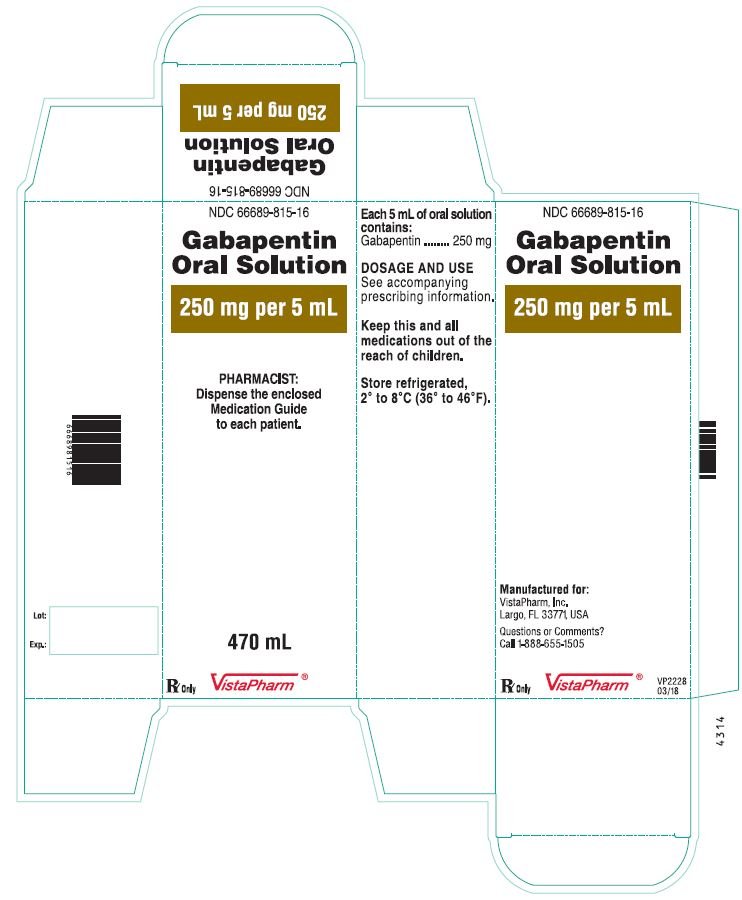
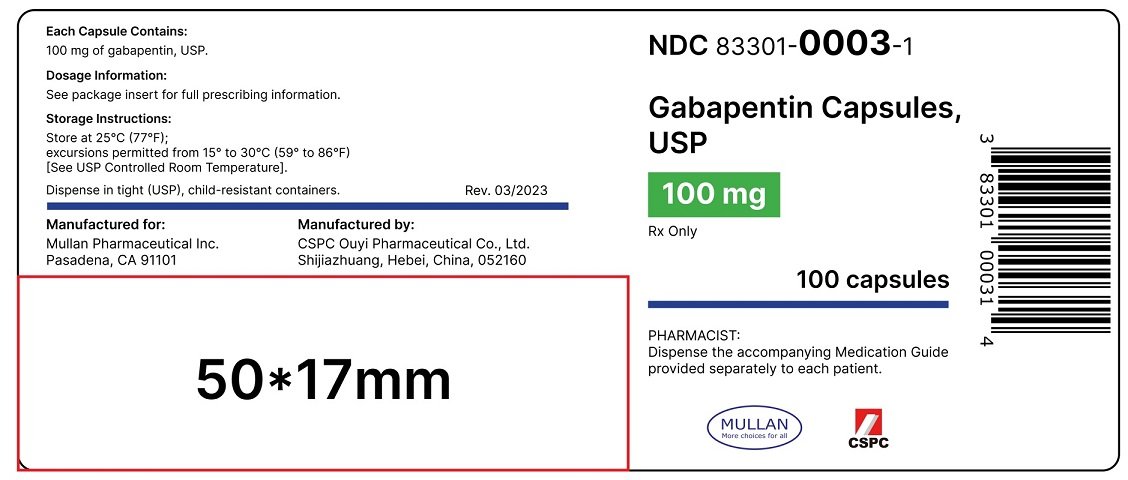
The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may be administered as the oral capsule. Gabapentin Oral Solution is a prescription medicine used to treat: • Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults ( 1) Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy ( 1) Page 2: Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the Page 8: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Package Description: Multilevel Packaging: 1: NDC Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. The NDC code 42192-608 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Acella Pharmaceuticals, Llc. The product's dosage form is solution and is administered via oral form. Do not stop taking gabapentin without first talking to your healthcare provider. Stopping gabapentin suddenly can cause serious problems. Gabapentin can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. GABAPENTIN IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS • Known sensitivity to Gabapentin or its ingredients WARNINGS AND PRECAUTIONS • Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan hypersensitivity): Discontinue if alterna-tive etiology is not established Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. The NDC Packaged Code 42192-608-16 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Acella Pharmaceuticals, Llc. The product's dosage form is solution and is administered via oral form. Order Gabapentin 250 mg / 5 mL Solution 470 mL by Acella Pharmaceuticals 42192060816 Acella Pharmaceuticals: Country of Origin: Unknown: Alternate Manufacturer Stopping gabapentin oral solution suddenly can cause serious problems. Gabapentin oral solution can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin oral solution may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Please see links below for Full Prescribing Information, including BOXED WARNING and Important Safety Information. To report suspected adverse reactions, contact the FDA at (800) FDA-1088 or www.fda.gov/medwatch. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and Do not stop taking Gabapentin Oral Solution without first talking to your healthcare provider. Stopping Gabapentin Oral Solution suddenly can cause serious problems. Gabapentin Oral Solution can cause serious side effects including: 1. Like other antiepileptic drugs, Gabapentin Oral Solution may cause suicidal thoughts or actions Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin Oral Solution is indicated for: • Management of postherpetic neuralgia in adults • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |