Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
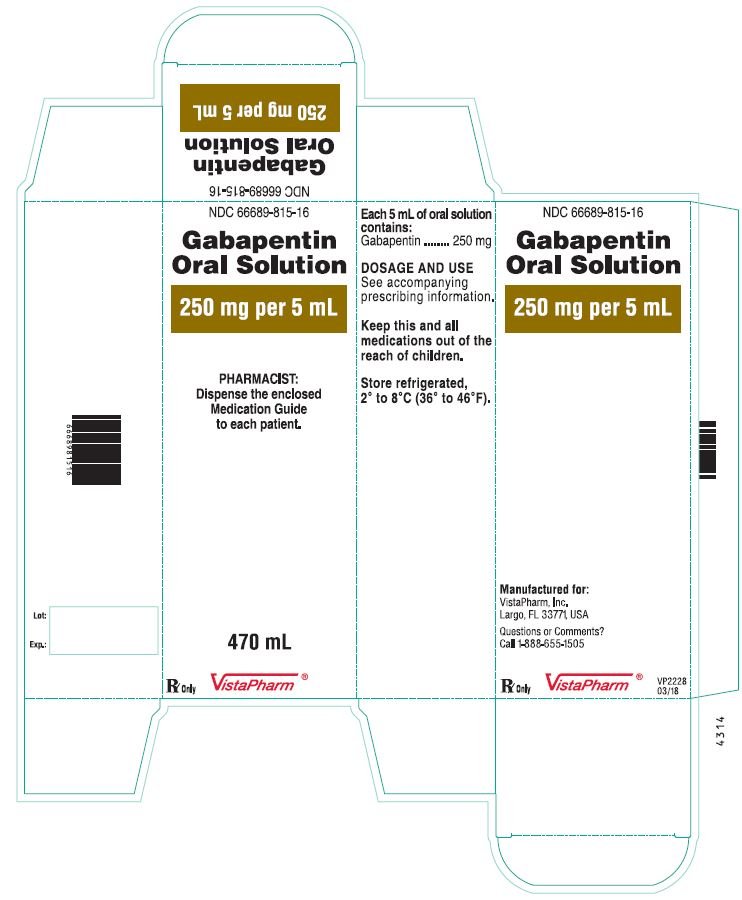
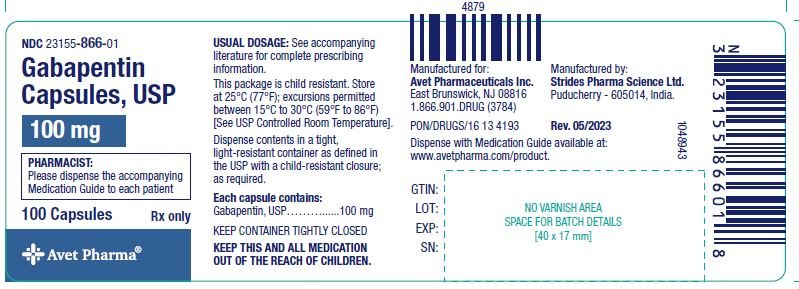
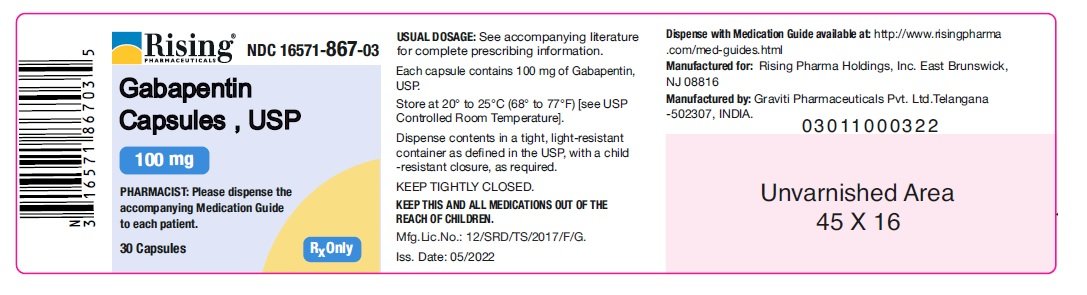
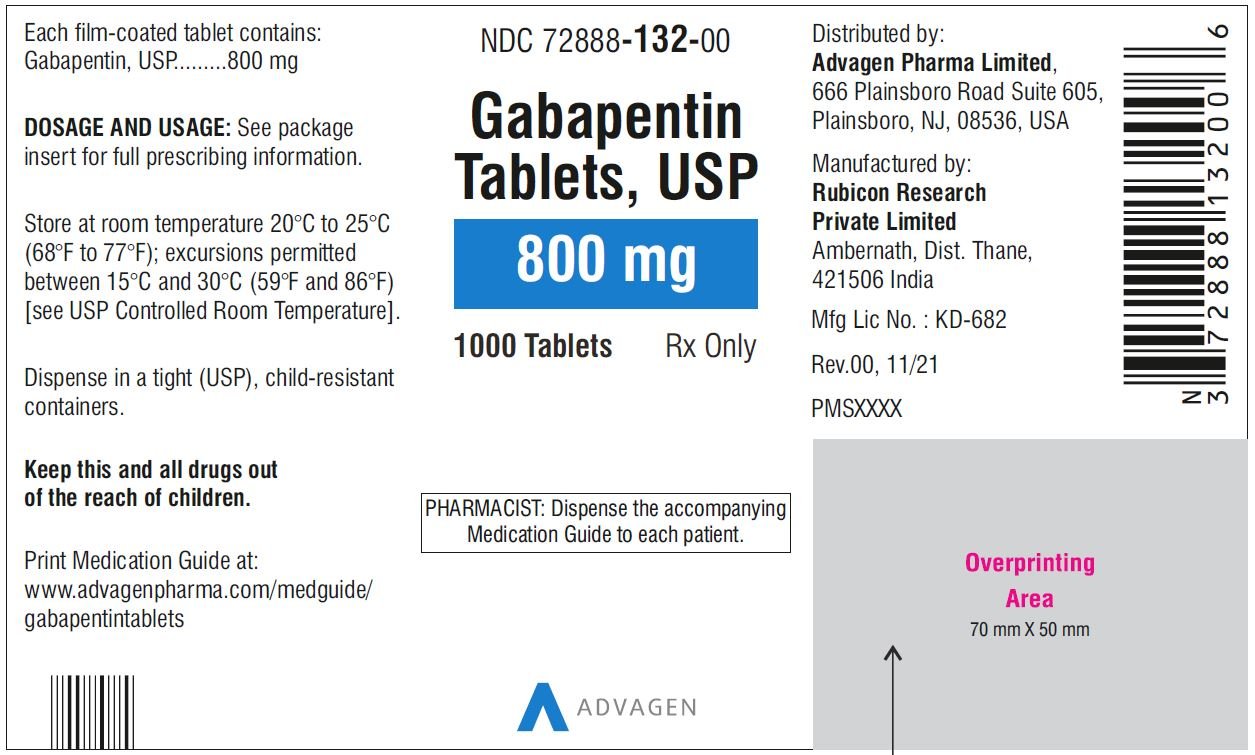
In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). Page 8: Quality Care Products, LLC: Gabapentin is indicated for: Management of postherpetic neuralgia in adults. Package Description: Multilevel Packaging: 1: NDC Do not stop taking gabapentin without first talking to your healthcare provider. Stopping gabapentin suddenly can cause serious problems. Gabapentin can cause serious side effects including: 1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. The precise mechanisms by which gabapentin produces its analgesic and antiepileptic actions are unknown. • Stopping gabapentin suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus). NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 GABAPENTIN IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS To report SUSPECTED ADVERSE REACTIONS, contact Acella Pharmaceuticals at 1-800-541-4802 Order Gabapentin 250 mg / 5 mL Solution 470 mL by Acella Pharmaceuticals 42192060816 Please see links below for Full Prescribing Information, including BOXED WARNING and Important Safety Information. To report suspected adverse reactions, contact the FDA at (800) FDA-1088 or www.fda.gov/medwatch. Acella Pharmaceuticals, LLC is a specialty pharmaceutical company committed to innovating the healthcare field by bringing quality and affordable products to our customers and patients. Our diverse portfolio of therapeutic products helps patients with a variety of healthcare needs. See full prescribing information for GABAPENTIN ORAL SOLUTION. Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily. Gabapentin may be administered as capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In elderly patients, and in patients with impaired renal function, gabapentin plasma clearance is reduced. Patients taking Gabapentin Oral Solution should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Gabapentin Oral Solution 300mg UD Cup 6mL 40/Package 1410602 | Acella Pharmaceuticals - 42192060840 The NDC Packaged Code 42192-608-16 is assigned to a package of 470 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Acella Pharmaceuticals, Llc. The product's dosage form is solution and is administered via oral form. What is Gabapentin Oral Solution? z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. z Partial seizures when taken together with other medicines in adults and children 3 years of age and older. Who should not take Gabapentin Oral Solution? Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and Gabapentin is a human prescription drug by Acella Pharmaceuticals, Llc. The product is distributed in 5 packages with NDC codes 42192-608-05, 42192-608-06, Page 2: Acella Pharmaceuticals, LLC: Gabapentin Oral Solution is indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |