Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
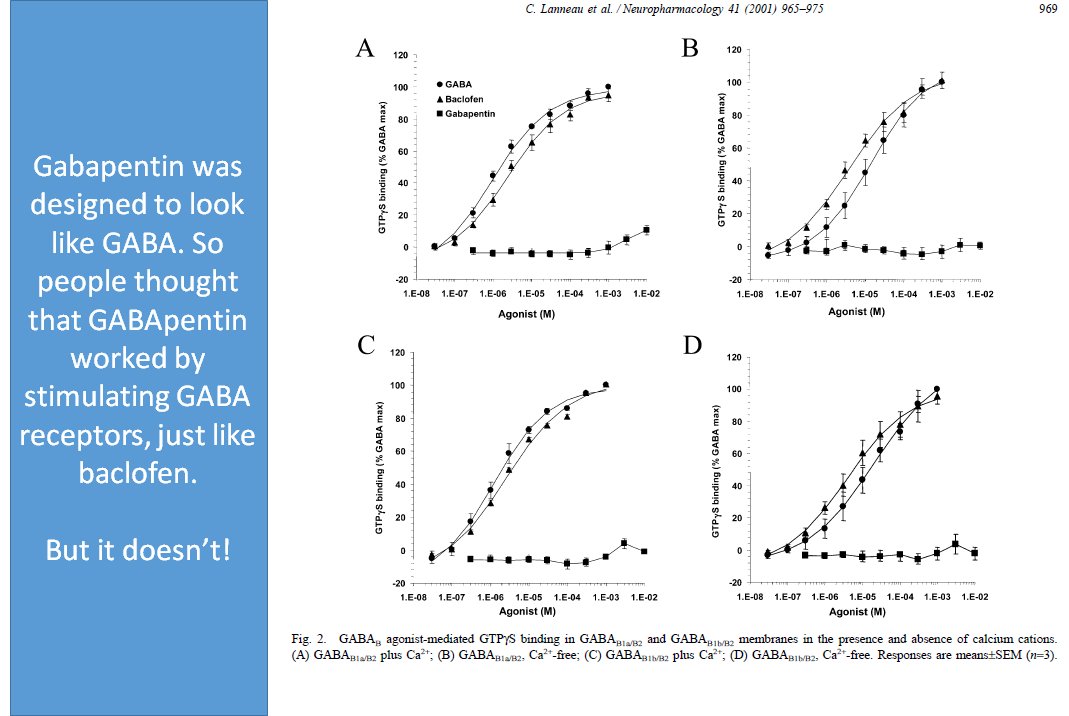
Finally, I downloaded the most recently updated US Food and Drug Administration (FDA)-approved product insert for gabapentin, which gained FDA approval in 1993, and its more potent successor, pregabalin, which was approved by the FDA in 2004. 7,8 Both drugs are available in generic formulations, and a 90-day supply of an average dose of either Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers. The main differences are their indications—specific uses that the Food and Drug Administration (FDA) has approved them to treat—and their dosages. Gabapentin and pregabalin are FDA-approved to treat some of the same conditions, including postherpetic neuralgia in adults. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10][7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] . Indications and Usage for Gabapentin. • Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. 2. Gabapentin Dosage and Administration. Gabapentin has been approved by the United States (US) Food and Drug Administration (FDA) for postherpetic neuralgia and as adjunctive therapy for focal seizures. 1 However, a recent analysis of US physician office-based prescription practices between 2011 and 2016 found that less than one percent of gabapentin prescriptions are for such indications. 2 In 2020, gabapentin was reported to be Gabapentin is FDA-approved as Neurontin to treat partial seizures in adults and children with epilepsy. Partial seizures are convulsions that originate from a single location in the brain. Neurontin is also approved to treat a type of nerve pain called postherpetic neuralgia, or PHN. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg gabapentin has been increasingly encountered by law enforcement, documented in national crime lab reports, reported to poison control centers, and diverted for illicit use. Licit Uses: According to the FDA-approved product label, gabapentin is used clinically for the management of postherpetic neuralgia in adults and as FDA-Approved Indications. Gabapentin: Gabapentin is indicated for postherpetic neuralgia and serves as adjunctive therapy for managing partial seizures (with or without secondary generalization) in adults and pediatric patients aged 3 or older. Other approved uses include fibromyalgia and restless legs syndrome. Gabapentin was first approved in 1993 and pregabalin was first approved in 2004. RECOMMENDATION: Gabapentin was first approved by the FDA on the basis of 3 multicenter, 12-week, double-blind, parallel-group trials that included a total of 705 adults with partial epilepsy and compared the effect of gabapentin vs placebo added to an existing antiepilepsy therapy. These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. These highlights do not include all the information needed to use GRALISE safely and effectively. See full prescribing information for GRALISE. GRALISE ® (gabapentin) tablets, for oral use Initial U.S. Approval: 1993 ----- Warnings and Precautions (5.2) 4/2020 ----- Gabapentin misuse and abuse have been reported in the postmarketing setting and published literature. Most of the individuals described in these reports had a history of polysubstance abuse. Some of these individuals were taking higher than recommended doses of gabapentin for unapproved uses. These highlights do not include all the information needed to use NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 When used as directed, gabapentin is known to have numerous uses and benefits. It has been FDA-approved to help control and treat seizures and to diminish a specific type of nerve pain called Owing to the multiple actions of the GABA system, gabapentin has subsequently been used for a wide variety of conditions, with up to 95% of gabapentin today prescribed for off-label indications. 2,3 Prescribers are often unaware of gabapentin’s approved indications and their prescribing of gabapentin is largely guided by informal discussion Gabapentin was first approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures in 1993 and was subsequently approved for one pain indication, postherpetic neuralgia.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |